4969
Synaptic Basis for Remodeling in the Mouse Somatosensory System Characterized by fMRIEmily Petrus1, Galit Saar1, Zhiwei Ma1, Steve Dodd1, and Alan Koretsky1
1Laboratory of Functional and Molecular Imaging, National Institutes of Health, NINDS, Bethesda, MD, United States
Synopsis
Functional MRI (fMRI) is an effective tool to analyze brain activity, with research and clinical implications. Here we describe changes in fMRI stimulus evoked BOLD responses after unilateral whisker denervation in adult mice. These effects were mediated by specific synaptic remodeling in the whisker somatosensory system. Our results indicate that fMRI can detect circuit level changes in vivo, which can then be characterized at the synaptic level in vitro.
Introduction
BOLD fMRI relies on the fact that neural activity causes increases in blood flow to local brain regions1. Previously our lab reported that unilateral whisker loss after infraorbital nerve transection (ION) produces an increase in BOLD fMRI response to the intact whisker set. This was reported to be mediated by a post-critical period re-opening of thalamocortical (TC) plasticity in the rat somatosensory barrel cortex (S1BC)2. We reproduced these effects in mice, which allows for further characterization of the system using additional tools such as optogenetics. Here we report the expected increase of intact S1BC, mediated by a stronger TC synapse. We also observed a similar bilateral response to unilateral stimulation of the intact whisker set in ION mice and rats. Finally, we report synaptic remodeling after whisker denervation occurs at the callosal input to the deprived S1BC, targeting a specific cell type.Methods
Adult mice underwent a unilateral sham or ION surgery, which removes input from one whisker pad. Two weeks after ION animals were anesthetized with an IP injection of a ketamine/xylazine mixture and imaged in an 11.7T animal MRI system (Fig. 1A) (30 cm horizonal bore, Magnex Scientific, Oxford, England; MRI Electronics, Bruker Biospin, Billerica, MA). Scan parameters were: TR/TE = 1000/15 ms, .22 mm in plane resolution, .4 mm slice thickness, 7 slices, matrix size 64 x 48. Two 30G needles were placed in the intact whisker pad and 2mA currents were delivered to elicit stimulus-evoked BOLD fMRI. A separate group of mice were unilaterally injected with channel rhodopsin (ChR2) into intact S1BC, underwent ION surgery, were euthanized, and brain slices were prepared for whole-cell slice electrophysiology (Fig. 1A). Principal neurons in the intact and deprived S1BC were patched and synaptic strength was measured between brain regions using optogenetics and strontium evoked desynchronized vesicle release, which enabled quantal analysis of post-synaptic strength.Results
Unilateral whisker stimulation evoked a reliable BOLD fMRI signal in the contralateral S1BC in sham animals, which was significantly larger in ION animals (Fig. 1B). This is mediated by a stronger TC synaptic connection in the ION group (Fig. 1D). Interestingly the unilateral stimulus provoked a bilateral BOLD response in ION animals (Fig. 1B). We determined that the bilateral activation is due to a stronger callosal connection from the intact to deprived S1BC, which specifically targeted layer 5 (L5) principal neurons (Fig. 2C). The callosum did not strengthen connections to layer 2/3 (L2/3) neurons (Fig. 2B) and local connections between L5 cells were similarly unaltered (Fig. 2D).Discussion
Neural circuits are known to be plastic in response to changes in sensory experience, even in adult organisms. Our experiments describe a change in the somatosensory circuit which we detected with BOLD fMRI, and then characterized at the synaptic level using whole-cell slice electrophysiology. It will be interesting to determine if the specific synaptic changes also lead to changes in global resting state fMRI in a manner similar to the effects we report in stimulus-evoked fMRI. This has implications for the detection and description of brain disorders in vivo which have a synaptic, circuit level change in function.Conclusion
Here we demonstrate that stimulus-evoked BOLD fMRI can detect changes in neural activity which can then be described at the cellular and synaptic level with whole cell electrophysiology. These results indicate that BOLD imaging can be a useful tool to identify locations of robust neural circuit reorganization.Acknowledgements
No acknowledgement found.References
1. Logothetis, NK. The Royal Society 2002; 3(1): 13-21.
2. Yu X, Chung, S, Chen DY, Wang S, Dodd SJ, Walters JR, Isaac JT, Koretsky AP. Neuron 2012; 74(4):731-42.
Figures
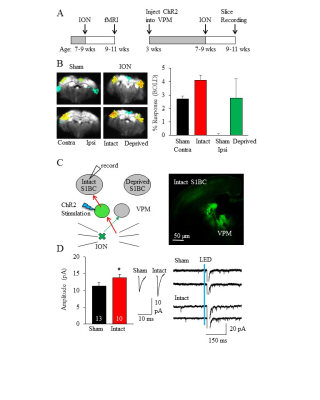
Figure
1: fMRI BOLD signal
in
S1BC increases in response to intact whisker stimulation after ION. (A)
Experimental timeline for fMRI (left) and whole cell recordings (right). (B)
Response to contralateral whisker stimulation is unilateral in sham, but is
bilateral in ION
animals. The intact S1BC also
demonstrates increased response compared to sham
contralateral stimulation. (C)
Experimental diagram (left) for whole cell recordings with corresponding brain
slice (right). The TC synapse from intact VPM to intact S1BC is stronger after
ION.
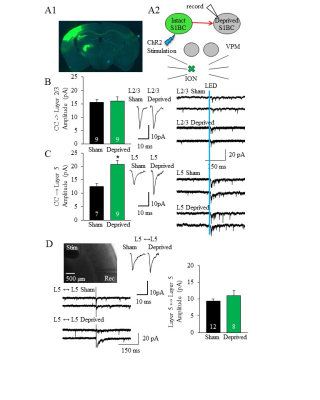
Figure
2: Corpus callosum strengthens synapses specifically to layer 5 (L5) principal
neurons after ION surgery. (A1) Brain slice demonstrating left S1BC injection
of ChR2-eYFP (green) with terminals to right S1BC, (A2) experimental schematic. (B) Synaptic
strength is not changed to layer 2/3 principal neurons, but doubled to L5 (C).
(D) Local connections between L5 neurons are not significantly different
between groups.