4965
Towards the Macaque Connectome: 24-Channel 3T Multi-Array Coil, MR Sequences, and HCP-style Preprocessing1Center for Life Science Technologies, RIKEN, Kobe, Japan, 2Takashima Seisakusho Co., Ltd, Tokyo, Japan, 3Siemens Healthcare Japan, Tokyo, Japan, 4Department of Neuroscience, Washington University, St. Louis, MO, United States, 5St. Luke's hospital, St. Louis, MO, United States
Synopsis
Macaque monkeys are an important neuroscientific model for
understanding cortical organization of primates, yet non-human primate MRI
applications have lagged behind those developed for humans. To address this
issue, we developed a 24-channel receive coil for macaque brain imaging and
adapted the Human Connectome Project (HCP)’s image acquisition protocols matched
to monkey’s neuroanatomical resolution. By adapting HCP’s preprocessing methods
for the macaque, we demonstrate that the resulting HCP-style monkey MRI data
show great promise for multi-modal analyses of cortical architecture and
connectivity, which have previously only been possible in humans.
Introduction
Macaque monkey is an important neuroscientific model for
understanding cortical organization of primates, yet MRI coils and protocols
for non-human primates have lagged behind those for humans1,2.
To resolve architectural and functional details of thin macaque cortex, high
spatial and temporal resolutions are required while maintaining high SNR. To
address this challenge, we developed a 24-channel receive coil optimized for macaque
brain scanning. The coil enabled adaptation of the Human Connectome Project
(HCP)-style image acquisition protocols1,3,4 to match smaller scale of macaque neuroanatomy. Furthermore,
we adapted the HCP’s preprocessing methods for the macaque, including pipelines
for structural, functional, and diffusion MRI and ICA+FIX denoising5. The
resulting HCP-style monkey MRI data show great promise for multi-modal analyses
of cortical architecture and connectivity, which have previously only been
possible in humans.
Methods
The coil frame geometry was designed using a 3D digital design
software (Rhinoceros 5, McNeel, USA) to closely fit the head geometry obtained
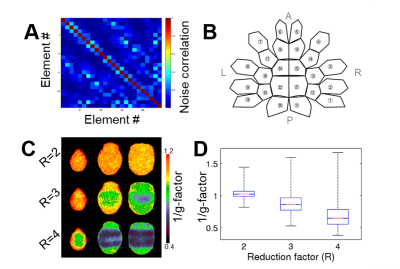
from the animals within our macaque MRI database (Fig. 1A). pentagonal and
hexagonal elements were configured over the surface (Fig. 1B,C and D),
resembling soccer-ball coil design6. The elements were
arranged in three arrays lined vertically in rostral-caudal direction to
maximize parallel encoding power of multiband EPI sequence in axial slice
direction.
Experiments were performed in clinical 3 T MRI scanner
(MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) equipped with 80 mT/m
gradients (XR 80/200 gradient system). To improve comparison between macaque
and human species, our data acquisition strategy was to obtain data following
methodologies introduced by the HCP (3 T protocols)1. In brief,
anatomical images were acquired using T1w MPRAGE (0.5 mm isotropic, TI 900 ms,
TR 2200 ms) and T2w SPACE (0.5 mm isotropic, TE 562 ms, TR 3200 ms, GRAPPA 2).
Resting-state fMRI was acquired twice, each different in phase-encoding direction
using gradient-echo EPI (1.25 mm isotropic, TE 30 ms, TR 755 ms) and comprised a
total of 100 minutes per animal. Diffusion scans were acquired 2D spin-echo EPI
(0.9 mm isotropic, TE 73 ms, TR 3200 ms, GRAPPA 2, multi-band factor (MBF) 2) with
monopolar gradient scheme (b-values 1000, 2000 and 3000 s/mm2 and 500 uniformly
distributed directions) resulting in a scan time of 30 min.
Data
analysis utilizes the non-human primate version of HCP (NHPHCP) pipelines including
structural (PreFreeSurfer, FreeSurferNHP, and PostFreeSurfer), functional
(fMRIVolume, fMRISurface) and diffusion preprocessing (DiffusionPreprocessing)1,7. These processes transformed the data into a standard
set of grayordinates (164k, 32k and 10k for structural, dMRI and fMRI,
respectively) in the dense Connectivity Informatics Technology Initiative
(CIFTI) format. In addition, fMRI was denoised using FIX (manual reclassification)
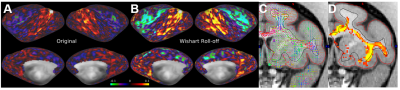
for structured artefacts5, and using Wishart roll-off for unstructured noise2. Diffusion MRI data was also used for voxel-wise estimation of
crossing fibers8 and surface-based probabilistic tractography7.
Results
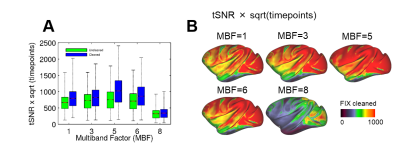
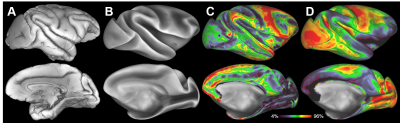
The parallel imaging capabilities of the coil were assessed using noise correlation (Fig. 2A,B), GRAPPA g-factor (Fig, 2C,D) and MBF (Fig. 3A,B) measures. Together, mean low noise correlation (8.4 ± 8.5% std) small noise cancellation through parallel reconstruction (1/g = 1.03 ± 0.07) and optimal MBF=5, indicate the benefit of accelerated imaging achieved through the geometrical arrangement of the coil elements. Strikingly, despite the 4-fold SNR penalty from spatial resolution (isotropic 1.25 mm macaque vs 2 mm HCP), the fMRI tSNR in macaque surpasses the HCP (49.7±18.4 vs 43.0±15.2), highlighting the advantage of proximity of the coil elements to cortex. The benefit of our methodology is further demonstrated by accurate segmentation of pial and white matter surfaces and generation of surface sheet (Fig. 4A,B), cortical thickness (Fig. 4C) myelin (Fig. 4D) maps, sensitive functional connectivity estimates (Fig. 5A,B) and identification of challenging crossing fiber pathways (Fig. 5C,D).Discussion & conclusions
Here, we presented a macaque multi-modal imaging methodology using combination of a custom-made 24 channel receive-coil, HCP-style high-resolution parallel imaging and the NHPHCP analysis pipelines. Combination of high-density coil with accelerated imaging was reasonably effective particularly for functional and diffusion acquisition. Although further optimizations are still needed, our methodology may enable not only more accurate multi-modal comparisons across macaque-subjects but also more direct inter-species comparison between macaque and human connectomes. We anticipate that this approach opens exciting research avenues into evolutionary expansion of cortical areas9, means to validate human neuroimaging technologies7 and investigations into connectome dynamics which may shape behavior. Overall, we've demonstrated that MRI studies in macaque may have compelling benefit from adapting the imaging methodologies introduced by the HCP.Acknowledgements
We thank Prof. Van Essen DC in Washington University School of Medicine for providing the Non-Human Primate Connectome Pipeline. This research is partially supported by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and development, AMED, by MEXT KAKENHI Grant (16H03300,16H03306, 16H01626, 15K12779).References
1Glasser et al., (2013) Neuroimage 80,
105–124. 2Glasser et al., (2016) Nature 536, 171-178. 3Smith et al., (2013) Neuroimage 80, 144-168. 4Sotiropoulos et al., (2013) Neuroimage 80, 125-143. 5Griffanti et al., (2014) Neuroimage 95, 232-247. 6Wiggins et al., (2006) Magn. Reson. Med. 56, 216-223. 7Donahue et al., (2016) Journal of Neuroscience, 36, 6758-70. 8Behrens et al., (2007) Neuroimage 34, 144-155. 9van
Essen et al., (2007) Neuron 56, 209-225.
Figures