4960
Assessment of metabolic changes in traumatic brain injury using hyperpolarized [1-13C]pyruvate: a longitudinal study1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Radiology, Loma Linda University, Loma Linda, CA, United States
Synopsis
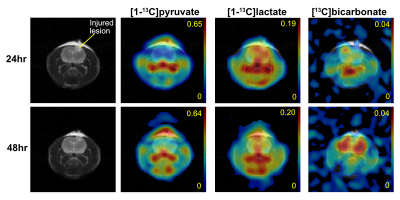
In this study, we longitudinally assessed acute metabolic changes during 2-120hrs post traumatic brain injury in a controlled-cortical impact rat model using hyperpolarized [1-13C]pyruvate. We observed mildly increased pyruvate conversion to lactate and significantly reduced bicarbonate production in the injured site at 24hrs after impact. Conversely, lactate was reduced when measured at 48hrs-post injury. Bicarbonate production in the lesion remained low with significantly increased bicarbonate production in the contralateral normal-appearing brain
Background
Traumatic brain injury (TBI) is one of the leading causes of death in the United States, contributing to approximately 30% of all injury-related deaths from 2002-20061. The primary tissue damage from the injury results in metabolic shifts to meet evolving energy demands, resulting in possible secondary damage2. This secondary damage is thought be lead to potential life-long disabilities, cognitive and memory impairments, and increased risk for mood disorders and neurodegenerative disease2-4. Several studies demonstrate dysregulation of cerebral energy metabolism in TBI5. Common features reported in the pathophysiology of TBI are 1) the increase of glucose consumption rate with no parallel increase in mitochondrial oxidative phosphorylation, known as hyperglycolysis, and 2) mitochondrial dysfunction. A recent study demonstrated the use of hyperpolarized [1-13C]-pyruvate as a noninvasive imaging biomarker for alterations of energy metabolism6; however, there is little information regarding the timing of early metabolic changes in the brain due to traumatic injury. In this study, we acquired metabolic snapshots at five different time-points (2-120hrs) after brain injury by administering hyperpolarized [1-13C]pyruvate in order to track metabolic changes over time and pinpoint the peak of early secondary metabolic damage.Methods
TBI was induced in male Wistar rats (n=6) using the controlled cortical impact model (CCI)7. Rats received a ~5mm diameter craniotomy at -2.0mm (anteroposterior) and +2.0mm (mediolateral) to bregma. The contusion was induced using a 3-mm cylindrical tip at a velocity of 4.40m/s, 2mm depth of tissue compression, and a dwell time of 100ms. All the imaging data were collected using on a GE 3T 750W wide-bore scanner and a 13C/1H dual-tuned birdcage RF coil. The rats were imaged at 2hr, 24hr, 48hr, 72hr, and 120hr post-injury. T2-weighted fast spin echo (FSE) images (TE/TR=11.3ms/5000ms, thickness=2.0mm, matrix=256x256, FOV= 9.6x9.6mm2) and FLAIR images (TE/TR=24ms/3000ms, thickness=2.0mm, matrix=384x384, FOV=9.6x9.6mm2) were acquired to identify location and volume of the injured area. A 35-μL sample of 14-M [1-13C]-pyruvate mixed with OX063 trityl (15mM) was prepared for each dissolution and polarized using a SPINlab clinical DNP polarizer. After 3-4hrs of polarization, pyruvate samples were dissolved, mixed with pH-neutralization media (NaOH), and immediately injected over 10-12s intravenously (70-mM pyruvate, ~7.5 of pH). 13C data was acquired using a free-induction decay chemical shift imaging (FID CSI; spectral width=5000Hz; spectral points=256; FOV=50x50mm2; matrix=16x16, thickness=7.7mm) sequence beginning 25s after the start of the injection. [13C]-labeled pyruvate, lactate, and bicarbonate levels were calculated from the averaged spectra within the regions of interest (ROIs) within the injured area and the non-impacted contralateral area. Each metabolite was normalized by the total 13C (TC) signal measured within the ROI. Evolution of metabolite ratios over the observation time window was evaluated using MANOVA and differences between the lesion and non-lesion ROI were evaluated using student t-tests.Results and Discussion
T2-weighted images revealed the presence of a contusion immediately (1-2hrs) after impact with increased edema near the impact site. The T2-hyperintense injured tissue increased after the impact and were peaked at 24-48hrs post-injury. No significant difference in [1-13C]lactate and 13C-bicarbonate maps were found at 2hrs-post TBI between injured and contralateral normal tissue. The greatest increase in [1-13C]lactate production in the lesion area was detected at 24hrs-post injury but did not reach statistical significance. In contrast, bicarbonate production in the lesion area was acutely and significantly reduced throughout the imaging window (24-120hrs), Fig.1. Both of the observations are consistent to literature8. The increased lactate production is related to upregulated lactate dehydrogenase (LDH), with the decreased bicarbonate conversion associated with mitochondrial dysfunction and deactivated pyruvate dehydrogenase (PDH)9. Interestingly, bicarbonate production in contralateral normal-appearing brain was significantly higher in 48hrs and 72hrs post-injury than earlier time points (2hrs and 24hrs). In addition, we found that for intra-subject temporal and inter-subject comparisons, the product-ratio was more consistent and reliable than individual metabolite levels (e.g., lactate/TC and bicarbonate/TC). This is probably because we used small volumes of pyruvate sample and dissolution media in a clinical polarizer, resulting in a relatively large discrepancy in the final outcome volume (6-8mL) and therefore, final pyruvate concentration. This study demonstrates the feasibility of hyperpolarized 13C-pyruvate MRS as a noninvasive tool for monitoring metabolic changes during the acute secondary injury processes following TBI. Considering the high prevalence of TBI and recent translational progress of this technology in human subjects10,11, our results present an exciting opportunity for immediate clinical impact.Conclusion
We presented temporal changes in brain metabolism investigated by hyperpolarized pyruvate. In particular, we observed significant changes in bicarbonate production in both damaged brain and contralateral normal-appearing brain regions. Future work will be focused on replicating these findings in larger study groups as well as sham-operated rats. Histology and enzyme analysis from selected time points will be performed to verify the in vivo findings.Acknowledgements
Funding: National Institutes of Health of the United States (P41 EB015908, S10 OD018468); The Mobility Foundation; The Texas Institute of Brain Injury and Repair.References
1. Taylor, C. A., Bell, J. M., Breiding, M. J. & Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ 66, 1–16 (2017).
2. Hill, C. S., Coleman, M. P. & Menon, D. K. Traumatic Axonal Injury: Mechanisms and Translational Opportunities. Trends Neurosci. 39, 311–324 (2016).
3. Zetterberg, H., Smith, D. H. & Blennow, K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol 9, 201–210 (2013).
4. Kolias, A. G., Guilfoyle, M. R., Helmy, A., Allanson, J. & Hutchinson, P. J. Traumatic brain injury in adults. Pract Neurol 13, 228–235 (2013).
5. Bartnik-Olson, B. L., Harris, N. G., Shijo, K. & Sutton, R. L. Insights into the metabolic response to traumatic brain injury as revealed by (13)C NMR spectroscopy. Front Neuroenergetics 5, 8 (2013).
6. DeVience, S. J. et al. Metabolic imaging of energy metabolism in traumatic brain injury using hyperpolarized [1-(13)C]pyruvate. Sci Rep 7, 1907 (2017).
7. Chen, Y., Mao, H., Yang, K. H., Abel, T. & Meaney, D. F. A modified controlled cortical impact technique to model mild traumatic brain injury mechanics in mice. Front Neurol 5, 100 (2014).
8. Bartnik, B. L. et al. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J. Neurotrauma 22, 1052–1065 (2005).
9. Corps, K. N., Roth, T. L. & McGavern, D. B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 72, 355–362 (2015).
10. Nelson, S. J. et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate. Sci Transl Med 5, 198ra108 (2013).
11. Cunningham, C. H. et al. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ. Res. 119, 1177–1182 (2016).