4958
Gray matter volume changes following Cranial Nerve Non-invasive NeuroModulation in patients with traumatic brain injuries1Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 3Department of Kinesiology, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
The main conventional approach for treating traumatic brain injury related gait and balance deficits has been through physical therapy, but few approaches have focused on brain based rehabilitation efforts that create direct neuroplastic changes. There remains a need for such an approach. Therefore, the goal of this study was: 1) to apply Cranial Nerve Non-Invasive NeuroModulation (CN-NINM) via the tongue in combination with multiple symptom-specific physical therapy exercises in patients with mild to moderate TBI, and 2) to investigate and quantify gray matter volume changes prior to and after intervention as well as their correlation with behavior.
Introduction
The primary conventional approach for treating traumatic brain injury (TBI) related symptoms of gait and balance deficits is physical therapy. However, few studies have examined the efficacy of brain based rehabilitation techniques that harness direct neuroplasticity changes1. There remains a need for such an approach. One pilot TBI study suggested that, in the absence of identifiable tissue damage, a combination of neurostimulation and rehabilitation that is both targeted and challenging will induce neuroplastic changes, reduce symptoms, and begin normalizing function2. These changes include rehabilitation and re-establishment of movement control, and cognition. Therefore, the goal of this study was: first, to apply Cranial Nerve Non-Invasive NeuroModulation (CN-NINM) via the tongue in combination with multiple symptom-specific physical therapy3 exercises in patients with mild to moderate TBI, and second, to investigate and quantify gray matter volume (GMV) changes from pre- to post-intervention and their correlation with behavior.Methods
9 patients with TBI (at least one year post-injury, mean age = 53.11, SD = 6.60, 3 male and 6 female) were recruited. The CN-NINM intervention training consisted of twice-daily in-lab training for two-weeks. The participants also received physical exercise training to develop improved motor coordination and mobility. Structural MRI scans on a 3T GE scanner were collected using the FSPGR BRAVO sequence (TR = 8.132 ms, TE = 3.18 ms, TI = 450 ms) over a 256 x 256 matrix and 156 slices (flip angle = 12°, FOV = 25.6 cm, slice thickness = 1 mm) before the first intervention (‘pre’) and then after the final (‘post’) intervention session. At the same time, all participants completed two tasks of Sensory Organization Test (SOT) and Dynamic Gait Index (DGI) before and after the week of twice-daily interventions. SOT (NeuroCom International, Clackamas OR, USA) is an objective, automated measure of sensory-motor integration that evaluates the functional contribution of the somatosensory, visual, and vestibular components of balance. DGI is a clinician-scored index of eight facets of gait--normal walking, changing speed while walking, head turns and up/down tilts while walking, turning and stopping, walking around and stepping over objects, and traversing stairs4. Preprocessing for GMV was performed using the Computational Anatomy Toolbox (CAT Version 12; https://www.nitrc.org/projects/cat/) and SPM (Version 12) with MATLAB R2015a. Bias field correction was applied to correct for MRI inhomogeneities; noise was removed and voxel intensities were normalized5; brain tissue was segmented and normalized into six different tissues classes (gray and white matters, cerebrospinal fluid, bone, other soft tissues, and air/background) using the modified unified segmentation approach implemented in SPM6. Images were transformed nonlinearly to standard stereotaxic space of Montreal Neurological Institute (MNI) and resliced to 2×2×2 mm using diffeomorphic registration algorithm (DARTEL)7,8,9 to CAT12’s default template (IXI555_MNI152)10,11. For GMV analysis, gray matter probability maps were multiplied by the non-linear component of the Jacobian determinant, and modulated gray matter probability maps were spatially smoothed with an 8 mm full-width at half-maximum (FWHM) Gaussian kernel. The paired t-test analysis (pre vs. post interventions) was used for GMV data. The statistical threshold was set to p < .05 and cluster size > 212 using the AlphaSim multiple comparison correction. Pearson r correlations were used to examine the relation between changes in GMV (post- minus pre-intervention) and changes in behavioral measures (SOT and DGI). The statistical package SPSS 22.0 was used for all analyses, p < .05.Results
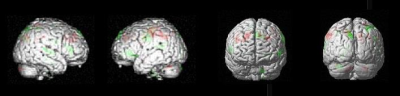
Paired t-test results showed significant increase from pre- to post-intervention on both the SOT and DGI. CN-NINM intervention induced multiple GMV increases within the temporal, frontal, occipital lobes and cerebellum, as well as some GMV decreases within the frontal and parietal lobes (Figure 1 and Table 1). Score differences on both SOT and DGI were negatively correlated to all GMV differences (Table 2).Discussion
These preliminary results suggest that overall there appears to be an increase in GMV of regions and possibly increase in functionality of regions involved in gait/posture/balance (cerebellum, associative areas in associative temporal-occipital regions) and decrease in GMV and possibly decrease in functionality of areas that were needed for compensation such as frontoparietal areas involved in attention/executive function.Conclusions
Overall regions involved in automatic processing of gait, balance and posture seemed to increase in GMV, whereas compensatory regions that were needed for effortful processing such as executive function and attention decreased in GMV. These results indicate that CN-NINM may be a promising way to treat the symptoms of TBI.Acknowledgements
This work was supported by the National Institute of Child Health and Human Development (grant number K12HD055894 to SS), and pilot funding from the UW-Madison Department of Radiology R&D (to SS) and the UW-Madison Department of Medicine (to SS), by the National Institute of Health (grant numbers T32GM008692, UL1TR000427, T32EB011434). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
1. Bach-y-Rita P. (1990). Brain plasticity as a basis for recovery of function in humans. Neuropsychologia, 1990;28(6) 547 – 554.
2. Danilov Y, Kaczmarek K, Skinner K, et al. Cranial nerve noninvasive neuromodulation: New approach to neurorehabilitation. In: Kobeissy FH, ed. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton. 2015.
3. Kaczmarek K. The Portable NeuroModulation Stimulator (PoNS) for NeuroRehabilitation. Scientia Iranica D. 2017;in press.
4. Shumway-Cook A, Woollacott M. Motor control: Translating research into clinical practice. 3rd edition. Lippincott, Williams, and Wilkins: Philadelphia. 2007.
5. Vovk U, Pernus , Likar B. A review of methods for correction of intensity inhomogeneity in MRI. IEEE Trans Med Imaging. 2007;26(3):405 – 421.
6. Malone I, Leung K, Clegg S, et al. Accurate automatic estimation of total intracranial volume: A nuisance variable with less nuisance. Neuroimage. 2015;104:366 – 372.
7. Killgore W, Olson E, Weber M. Physical exercise habits correlate with gray matter volume of the hippocampus in healthy adult humans. Scientific Reports. 2013;3:3457.
8. Loh K, Kanai R. Higher media multi-tasking activity is associated with smaller gray-matter density in the anterior cingulate cortex. PLoS One, 2014;9(9):e106698.
9. Michael A, Evans E, Moore, G. Influence of group on individual subject maps in SPM voxel based morphometry. Front. Neurosci. 2016;10:522.
10. Patil I, Zanon M, Novembre G, et al. Neuroanatomical basis of concern-based altruism in virtual environment. Neuropsychologia. 2017;S0028-3932(17)30061-1.
11. Wu J, Wu H, Yan C, et al. Aging-related changes in the default mode network and its anti-correlated networks: A resting-state fMRI study. Neuroscience Letters. 2011;504(1): 62 – 67.
Figures