4957
Transient disappearance of traumatic microbleeds on susceptibility weighted imaging in rats1Department of Radiology, University of Pécs, Pécs, Hungary, 2Department of Neurosurgery, University of Pécs, Pécs, Hungary, 3Department of Biochemistry an Medical Chemistry, University of Pécs, Pécs, Hungary, 4University of Pécs, Pécs, Hungary
Synopsis
Traumatic microbleeds (TMBs) are regarded as markers of traumatic brain injury (TBI) related diffuse axonal injury (DAI). According to several recently published observations, TMBs depicted by susceptibility weighted imaging (SWI), seems to show temporal changes. This study aims to explore the temporal features of TMBs in a rat model.
INTRODUCTION
Traumatic microbleeds (TMBs), most sensitively depicted by susceptibility weighted imaging (SWI), are regarded surrogate markers of diffuse axonal injury1,2. The extent of TMBs were shown to correlate with injury severity and outcome3,4. However, TMB appearance does not seem to be constant over time based on a number of previous case reports5,6.
METHODS
To elicit microbleed formation, after accessing the dura mater, brains of male Wistar rats (350 g – 450 g) were pierced in a depth of 4 mm, through the parietal cortex in a parasagittal position bilaterally using a stainless steel wire with a diameter of 159 μm (left side) or 474 μm (right side). Rats underwent 4.7T MRI immediately, then at 24h, 48h and 110 h after injury, including T1-, T2-, weighted imaging and 3D SWI. Coronal minimal intensity projection (mIP) reconstructions from 2 mm wide SWI volumes including the entire lesions were created. Microbleed volumes were measured using semi-automated intensity threshold based tracing (ImageJ7). As a control, region of interests were placed on adjacent unaffected cortical/white matter areas and were thresholded with the same intensity value. Microbleed volumes across time points were compared using repeated measures ANOVA with Bonferroni correction (Medcalc8, Ostend, Belgium). All procedures were carried out on rats in Isoflurane anesthesia, in full accordance with the ethical guidelines relating to animal research approved by the Animal Care Committee at the University of Pécs.
RESULTS
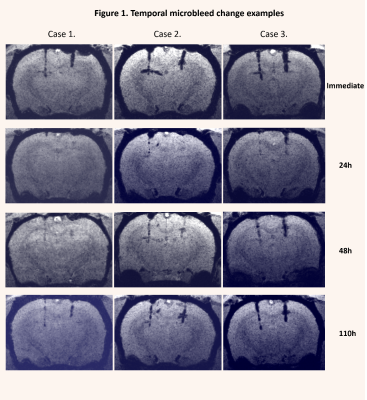
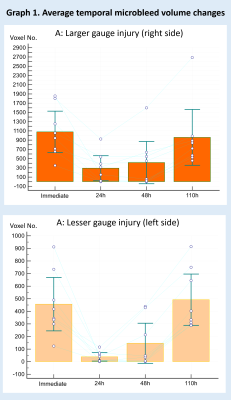
Microbleeds were detectable in SWI images but not in T1 or T2 images. Average microbleed volumes were significantly smaller (corrected p < 0.05) at 24h and 48h compared to the immediate and the 110h acquisition (graph 1. A and B). By visual inspection, several microbleeds detectable at the immediate and 110h imaging were completely missing or just slightly visible in the 24h and 48h images (figure 1). No apparent temporal changes at lesion sites were detected in the T1 or T2 images. The number of voxels within microbleed intensity range in the control region of interests showed no significant changes over time.
DISCUSSION
Our study showed a transient volume reduction, -or even complete disappearance of TMBs at 24h and 48h after injury in rats. This may be explained by a transient decrease of the susceptibility of hemoglobin degradation products over time. Our results may explain the previous human case reports of temporal microbleed changes.CONCLUSION
This phenomenon should not be interpreted as TMB regression (to disappearance) or progression (to re-appearance) and should be taken into account when applying SWI as a diagnostic or prognostic tool in traumatic brain injury.Acknowledgements
This study was funded by Hungarian Brain Research Program - No. KTIA_13_NAP-A-II/8, Grant No. KTIA_13_NAP-A-II/9, SROP-4.2.2.A-11/1/KONV-2012-0017 and Hungarian Scientific Research Fund Grant No. OTKA K 120356. The present scientific contribution is dedicated to the 650th anniversary of the foundation of the University of Pécs.References
1. S. Mittal, Z. Wu, J. Neelavalli, E.M. Haacke, Susceptibility-weighted imaging: technical aspects and clinical applications, part 2, AJNR Am J Neuroradiol 30 (2009) 232-252.
2. A. Di Ieva, T. Lam, P. Alcaide-Leon, A. Bharatha, W. Montanera, M.D. Cusimano, Magnetic resonance susceptibility weighted imaging in neurosurgery: current applications and future perspectives, J Neurosurg (2015) 1-13.
3. G. Spitz, J.J. Maller, A. Ng, R. O'Sullivan, N.J. Ferris, J.L. Ponsford, Detecting lesions after traumatic brain injury using susceptibility weighted imaging: a comparison with fluid-attenuated inversion recovery and correlation with clinical outcome, J Neurotrauma 30 (2013) 2038-2050.
4. E.L. Yuh, P. Mukherjee, H.F. Lingsma, J.K. Yue, A.R. Ferguson, W.A. Gordon, A.B. Valadka, D.M. Schnyer, D.O. Okonkwo, A.I. Maas, G.T. Manley, T.-T. Investigators, Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury, Ann Neurol 73 (2013) 224-235.
5. Toth A, Kovacs N, Tamas V et al. Microbleeds may expand acutely after traumatic brain injury. Neuroscience letters 2016;617;207-212.
6. Watanabe J, Maruya J, Kanemaru Y, Miyauchi T, Nishimaki K. Transient disappearance of microbleeds in the subacute period based on t2*-weighted gradient echo imaging in traumatic brain injury. Acta Neurochir (Wien) 2016;158;1247-1250.
7. Schneider CA, Rasband WS, Eliceiri KW. Nih image to imagej: 25 years of image analysis. Nature methods 2012;9;671-675. 8. Schoonjans F, Zalata A, Depuydt CE, Comhaire FH. Medcalc: A new computer program for medical statistics. Computer methods and programs in biomedicine 1995;48;257-262.
8. Schoonjans F, Zalata A, Depuydt CE, Comhaire FH. Medcalc: A new computer program for medical statistics. Computer methods and programs in biomedicine 1995;48;257-262