4955
Altered Functional Connectivity and Network Topology in Active Profession FightersKarthik R Sreenivasan1, Zhengshi Yang1, Virendra Mishra1, Sarah Banks1, Dietmar Cordes1,2, and Charles Bernick1
1Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, United States, 2University of Colorado Boulder, Boulder, CO, United States
Synopsis
Studies have shown that both active and retired athletes with repeated head trauma are more likely to suffer from cognitive decline and loss of executive and attention functions when compared to age- matched healthy controls. Our results show decreased functional connectivity in the active fighter group when compared to controls between regions known to be implicated in traumatic brain injury. Furthermore, we found a shift towards a less efficient network topology with altered integration and segregation in active professional fighters.
Introduction
Studies have shown that both active and retired athletes with repeated head trauma are more likely to suffer from cognitive decline and loss of executive and attention functions when compared to age- matched healthy controls [1,2]. The professional fighters brain health study (PFBHS) is a longitudinal study of active professional fighters [3]. In this study we use resting-state functional connectivity and graph theory to determine how the topology of the network is altered in active fighters with respect to healthy controls.Methods
18 boxers (28.72±7.38years) and 18 controls (29.16±8.57years) participating in the PFBHS were included in this study. Scan was conducted in Siemens 3T scanner (Siemens AG, Erlangen, Germany) using 32 channel head coil. The following MR parameters are used for resting state fMRI data acquisition; FOV=24×24 cm2 , matrix=128×128, flip angle = 80°, TR/TE = 2800 / 28ms, 4mm thickness, 30 slices. After standard preprocessing, mean time series were obtained from 90 ROIs based on the AAL atlas (excluding cerebellum and vermis). The connectivity between two ROIs was estimated using Pearson’s correlation between their averaged time-series, and subsequently a connectivity matrix (90 x 90) was obtained for each subject. Two-sample t-tests were used to compare the functional connectivity between the two groups regressing out age. In addition, these connectivity matrices were used to study the topological properties of the brain functional networks. Graph theory measures were obtained using GRETNA toolbox [4]. Path-length (Lp), clustering coefficient (Cp), Global (GE) and local efficiency (LE), were computed for each subject at various sparsity thresholds (S 0.05-0.4, ∆S=0.01). Two-sample t-tests with age as covariate were used to see if area under the curve for any of the above metrics were significantly different between the two groups.Results
Fig.1 shows a set of 40 enhanced connections in control group comparing with fighters, primarily comprising the putamen, hippocampus, frontal and temporal lobe and few other regions. Results were visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/) [4]. No paths were significantly greater in the fighters. The evaluation of the AUC values revealed significantly (p<<0.05) lower Lp (Fig. 2a) in the control group when compared to fighters but the Cp was not significantly different between the two groups. The area under the curve for GE (Fig. 2c) and LE (Fig. 2d) were also significantly (p<<0.05) greater in the controls when compared to the active fighters.Discussion and Conclusion
The current study revealed altered functional connectivity and topological properties of networks in active fighters compared to the healthy controls. Our results show decreased functional connectivity in the active fighter group when compared to controls between regions known to be implicated in traumatic brain injury. Furthermore, the fighter group showed a reduced GE and LE and increased Lp when compared to controls. These observations point to a shift towards a less efficient network topology with altered integration and segregation in active professional fighters.Acknowledgements
This work was supported by COBRE 1P20GM109025 and grants from Lincy foundation. We would like to extend our sincere thanks to all the participants of the study, various research coordinators and MRI technologists without which the study would not have been completed. We would also like to thank Dr. Mark Lowe and Dr. Wanyong Shin from Cleveland Clinic for their assistance in setting up the MRI protocols at our center.References
[1] McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013; 136: 43–64. [2] IR C, Siegel O, Sham R, EA C, Tarlau M, DiDomenico A. Brain damage in modern boxers. JAMA 1984; 251: 2663–7. [3] Bernick C, Banks S, Phillips M, et al. Professional fighters brain health study: Rationale and methods. Am J Epidemiol 2013; 178: 280–6. [4] Wang J, Wang X, Xia M, Liao X, Evans A and He Y (2015), GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci., 30 June 2015 | http://dx.doi.org/10.3389/fnhum.2015.00386 [5] Xia M, Wang J, He Y (2013) BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE 8(7): e68910. doi:10.1371/journal.pone.0068910Figures
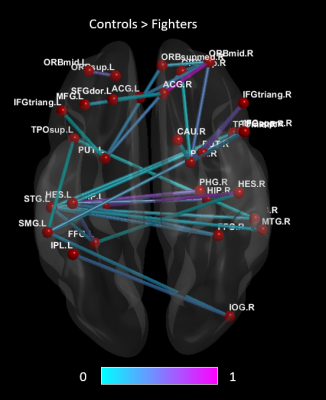
Figure 1. Shows paths that are
significantly greater
in healthy controls compared to the active professional fighters. The color
of the paths indicate least significant (0) to most significant (1).
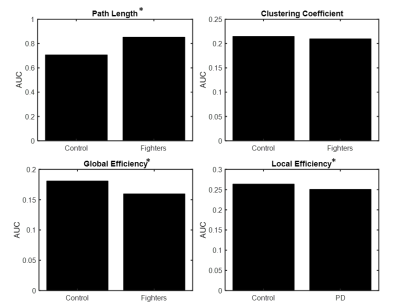
Figure
2 Mean age adjusted area under
the curve(AUC) for (a)
Path length (Lp) (b) Clustering
coefficient (Cp) (c)
Global efficiency (GE) and (d) Local
efficiency (LE) for both
controls and PD. *
indicates significant (p<0.05) difference.