4949
Functional correlates of microstructural damage in developmental traumatic brain injury: a multi-modal MRI study of neuroanatomy, cerebrovascular reactivity, and functional connectivity1Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Pharmacology, Physiology and Neurosciences, Rutgers New Jersey Medical School, Newark, NJ, United States, 3Radiology, Rutgers New Jersey Medical School, Newark, NJ, United States
Synopsis
The developing brain is particularly vulnerable to traumatic brain injury (TBI), with symptoms and co-morbidities lasting into adulthood. Animal models of developmental TBI are useful tools for investigating molecular pathways of TBI injuries and to assess novel therapeutics; however, validation of in vivo injury biomarkers in these models is important for the translational application of pre-clinical findings. Here, we demonstrate that the persistent dysfunctions of developmental TBI in an animal model can be measured at the level of microstructural damage (with diffusion MRI), functional interhemispheric connectivity (with resting-state fMRI) and systemic neurovascular coupling (with CO2 challenge fMRI).
Background
Mild-to-moderate traumatic brain injury (mTBI) is associated with a number of co-morbidities including cognitive deficits, posttraumatic epilepsy and increased risk of neurodegenerative diseases [1]. Developing brains are particularly vulnerable to the long-term consequences of mTBI, likely due to the higher proportion of excitatory synapses in high-plasticity developing brains which makes them more susceptible to secondary excitotoxic damage [2]. As such, management of TBI in children and teenagers poses some specific challenges. To this end, animal models of mTBI have been successfully used to investigate the cellular and molecular pathways involved in mTBI pathology. However, linking these findings with non-invasive biomarkers is crucial to generate translational knowledge that can be used in clinical mTBI. Here, we aim to investigate the long-term impacts of developmental mTBI using in vivo imaging biomarkers of structural fiber integrity (diffusion MRI), synaptic function (resting-state fMRI connectivity) and cerebrovascular reactivity.Methods
A sample of 17 male Sprague-Dawley rats aged 29-32 days were used for this study. All animals underwent a 3mm craniotomy on the left side of the skull, and a Luer-Lock syring hub was glued surrounding the exposed dura. 24 hours later, TBI was induced (n = 8) using a 20ms pendulum impact using a Fluid Percussion Injury (FPI) device. The sham group (n = 9) were fixed to the device without the pendulum drop. Imaging was performed 8-10 weeks later using a Bruker 9.4T spectrometer and an ellipsoidal surface coil (5 x 3 cm). Animals were anesthetized using urethane (1.3 mg/kg body weight, intraperitoneal), and body temperature was monitored throughout the procedure and maintained at 35-37ºC. DTI was acquired using a 4-segment EPI sequence with 5 A0 images, 30 diffusion directions and a b-value of 1000 s/mm2. Functional MRI images were acquired with single-segment EPI (TR/TE= 1000/15 ms); resting-state for 5 minutes repeated 4 times per animal, and cerebrovascular reactivity challenges for 12 minutes repeated twice, with 10% CO2 added to the breathing gas mixture between minutes 3 and 6 of the acquisition.Results
Voxel-level linear model of DTI parameters revealed lower fractional anisotropy (FA) in the white matter of mTBI animals, including the corpus callosum as well as the cingulum (in the somatosensory cortex area) and internal capsule ipsilateral to the injury (Figure 1A-B). Additionally, mean diffusivity (Apparent diffusion coefficient, ADC) was increased in mTBI animals in the corpus callosum and ipsilateral cingulum (Figure 1C-D). This impaired integrity of white matter tracts, particularly the corpus callosum, could indicate impairments in interhemispheric connectivity. To confirm this, resting-state functional connectivity of seed points in the cortex, dorsal hippocampus and thalamus contralateral to the injury was measured. All three structures showed significantly lower interhemispheric BOLD correlations in the mTBI animals compared to the sham group (Figure 2). Lastly, global cerebrovascular changes assessed with C02 challenge revealed a lower bilateral BOLD changes subcortically in the mTBI group, especially in the hippocampus and ventral thalamus (Figure 3).Discussion
Developmental mTBI was sufficient to induce diffuse and persistent deficits observable in vivo, at the levels of structural fiber integrity, functional interhemispheric networks, and systemic cerebrovascular reactivity. These findings, coupled with the lasting behavioral impairments observed in this model [3], appear to recapitulate the persistent vulnerabilities observed in children and adolescent concussive TBI patients. This model of local and diffuse developmental injury provides an ideal translational platform for the development of treatments beyond neuroprotection.Acknowledgements
No acknowledgement found.References
1. Chauhan, N.B., Chronic neurodegenerative consequences of traumatic brain injury. Restor Neurol Neurosci, 2014. 32(2): p. 337-65.
2. McDonald, J.W., F.S. Silverstein, and M.V. Johnston, Neurotoxicity of N-methyl-D-aspartate is markedly enhanced in developing rat central nervous system. Brain Res, 1988. 459(1): p. 200-3.
3. Murugan, M., V. Santhakumar, and S.S. Kannurpatti, Facilitating Mitochondrial Calcium Uptake Improves Activation-Induced Cerebral Blood Flow and Behavior after mTBI. Front Syst Neurosci, 2016. 10: p. 19.
Figures
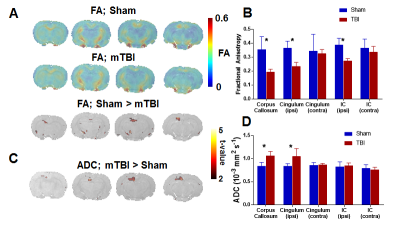
A) Average fractional anisotropy is projected on coronal slices for the Sham and mTBI groups, with voxel-level t-test indicating the areas of significant group differences. B) White matter ROIs show lower average anisotropy in the cingulum and internal capsule ipsilateral to the injury, as well as in the corpus callosum, indicating losses in white matter integrity. C) Voxel-level differences in mean diffusivity, as measured by the ADC. D) ROI analysis shows mTBI having higher diffusivity in the corpus callosum and ipsilateral cingulum, likely representing neuronal loss in these areas.
IC: Internal Capsule; * Significant difference at p < 0.01
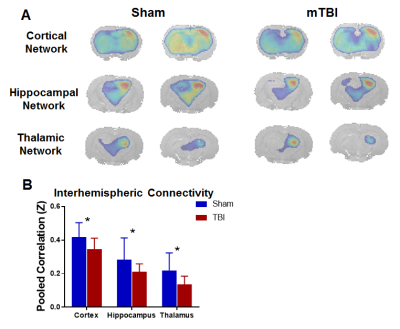
A) Group-level correlation with right-hemisphere (contralateral) cortical, hippocampal and thalamic seed points projected on coronal slices, for Sham and mTBI groups. B) Interhemispheric connectivity, as defined as the pooled correlation between the seed points and a corresponding hemisphere in the left hemisphere (ipsilateral to the lesion), is lower in mTBI for all three networks, confirming functional impairments in interhemispheric connectivity.
* Significant difference at p < 0.05
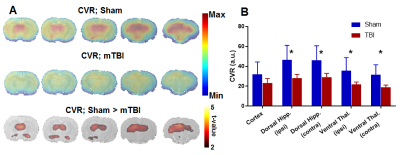
A) Changes in BOLD signal increase during CO2 challenge relative to baseline, shown as average maps for Sham and mTBI groups, as well as voxel-level significant differences. B) CO2-induced BOLD changes are globally lower for mTBI animals in subcortical ROIs, including hippocampus and thalamus in both the contralateral and ipsilateral hemispheres.
* Significant difference at p < 0.05