4948
Using fMRI to assess cerebrovascular reactivity after acute concussion1Neuroscience Research Program, St. Michael's Hospital, Toronto, ON, Canada, 2University of Toronto, Toronto, ON, Canada, 3Sunnybrook Hospital, Toronto, ON, Canada, 4St. Michael's Hospital, Toronto, ON, Canada
Synopsis
Cerebrovascular reactivity (CVR) is an important biomarker of concussion, as brain activity and blood flow regulation are often impaired after brain injury. This study examines an fMRI breath hold paradigm as a probe of CVR in concussed athletes, showing significant variations with days post-injury.
Objective
Concussion is a major public health concern and one of the most prevalent neurological conditions worldwide. It is mainly considered a functional disturbance, leading to altered neural metabolism and cerebral autoregulation (Giza & Hovda, 2001; Len et al., 2011). MRI studies have shown altered resting brain function (e.g., Churchill et al., 2017), but some studies have suggested that imaging the neurovascular response to physiological stress may be a more sensitive probe of concussion (Zhang et al., 2012). In this study, we assess cerebrovascular reactivity (CVR) of the brain using fMRI combined with a breath-holding and paced breathing paradigm. Our aim is to evaluate the feasibility of this paradigm in recently concussed individuals, and determine whether it distinguishes concussed individuals from uninjured controls.
Methods
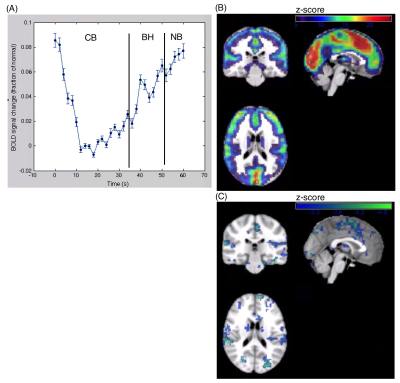
For this study, 18 varsity athletes were recruited from a university setting following a physician diagnosis of concussion, and scanned within 1 to 7 days post-injury. In addition, 30 control athletes without recent concussion were scanned. Participants were imaged using a 3T MRI and 20-channel head coil. The fMRI data were obtained via multi-slice T2*-weighted echo planar imaging (FOV = 20 × 20 cm, 64 × 64 matrix, 32 slices, 3.125 × 3.125 × 4.5 mm, BW = 2232 Hz/Pixel, TE/TR=30/2000, FA=70°, oblique axial interleaved). The paradigm consisted of a paced breathing block (PB) of 36 s, a breath hold block (BH) of 16s, followed by a normal breathing block (NB) of 8s. Data processing and analysis were performed using software from AFNI (afni.nimh.nih.gov) and customized algorithms developed in the laboratory. This included rigid-body motion correction, removal of outlier scan volumes, slice-timing correction, spatial smoothing with a 6 mm FWHM Gaussian kernel, regression of motion parameters and linear-quadratic trends as nuisance covariates. To control for physiological noise due to heartbeat and respiration, data-driven physiological correction was performed, along with regression of white matter signal. The fMRI data were then co-registered to a common anatomical reference using a series of affine transforms. At the individual subject level, we measured the mean change at the end of BH relative to CB. We then performed group-level analysis, comparing (a) the difference between acutely concussed athletes and controls, along with (b) the regressing activity against time post-injury in a general linear model (GLM).Results
Acute differences in BH response were highly variable across individuals, with concussed athletes showing no consistent mean difference relative to controls. However, regressing number of days post-injury against BH activity revealed a significant relationship: for athletes scanned within 1-3 days post-injury, significantly elevated BH response is seen, whereas for athletes scanned 4-7 days post-injury reduced BH response is observed. These findings indicate a differential acute response to concussion, which depends on the time post-injury at which they are scanned.
Conclusions
This study investigated fMRI with a breath-hold paradigm as a potential probe for changes in CVR among individuals with acute concussion. We demonstrate evidence of a reliable BH response that covaries with time post-injury, demonstrating the potential utility of this paradigm for characterizing neurovascular response to concussion.
Acknowledgements
Canadian Institutes of Health Research (CIHR)
Defence Research and Development Canada (DRDC)
Siemens Canada, Ltd.
References
Giza, C.C. and Hovda, D.A., 2001. The neurometabolic cascade of concussion. Journal of athletic training, 36(3), p.228.Vancouver
Len, Trevor K., J. Patrick Neary, Gordon JG Asmundson, David G. Goodman, Bruce Bjornson, and Yagesh N. Bhambhani. "Cerebrovascular reactivity impairment after sport-induced concussion." Medicine & Science in Sports & Exercise 43, no. 12 (2011): 2241-2248.
Churchill, N.W., Hutchison, M.G., Richards, D., Leung, G., Graham, S.J. and Schweizer, T.A., 2017. The first week after concussion: Blood flow, brain function and white matter microstructure. Neuroimage: clinical, 14, pp.480-489.
Zhang, K., Johnson, B., Gay, M., Horovitz, S.G., Hallett, M., Sebastianelli, W. and Slobounov, S., 2012. Default mode network in concussed individuals in response to the YMCA physical stress test. Journal of neurotrauma, 29(5), pp.756-765.
Figures