4946
Working Memory And White Matter Microstructure In Mild Traumatic Brain InjurySohae Chung1, Els Fieremans1, Xiuyuan Wang1, Charles J Morton1, Dmitry S Novikov1, Joseph F Rath2, and Yvonne W Lui1
1Center for Advanced Imaging Innovation and Research (CAI2R) & Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, NYU School of Medicine, New York, NY, United States, 2Department of Rehabilitation Medicine, NYU School of Medicine, New York, NY, United States
Synopsis
Working memory is a critical cognitive function implicated after mild traumatic brain injury (MTBI). Here, we investigate the association between white matter microstructure and working memory in normal controls (NC) and MTBI patients, using diffusion white matter tract integrity (WMTI) and WAIS-IV subtests, respectively. For the NC group, significant correlations were observed in axonal water fraction (AWF; higher axonal density/myelination) and mean kurtosis (MK; greater tissue complexity) with letter-number sequencing (LNS). However, such relationships were not present in the MTBI group.
INTRODUCTION
Working
memory is at the core of many critical cognitive functions, responsible for
holding, processing and manipulating information.1 Impaired working
memory is associated with a range of neurological disorders2 including
mild traumatic brain injury (MTBI).3 Little is known about whether
and how working memory relates to underlying white matter (WM) microstructure.
In this study, we investigate the association between WM microstructure and
performance on a set of working memory tasks in healthy adults and patients
with MTBI. We relate compartment specific WM tract integrity (WMTI) metrics4
derived from multi-shell diffusion MRI as well as diffusion tensor/kurtosis
imaging (DTI/DKI) metrics to Wechsler Adult Intelligence Scale-Fourth Edition
(WAIS-IV)5 subtests tapping auditory-verbal working memory.METHODS
We
studied 18 patients with MTBI (mean age, 30±7, range 22-45 yrs; 8 male) within a
month of injury (mean, 17 days) and 15 normal controls (NC) (mean age, 31±7,
range 19-45 yrs; 7 male). All subjects are right-handed and native English
speakers. MR imaging was performed on a 3T MR scanner (Skyra, Siemens). Diffusion
imaging was performed with 5 b-values (0.25,1,1.5,2,2,2.5ms/µm2) along with 6,20,20,30,60 diffusion
encoding directions using multiband (factor of 2) echo-planar imaging for
accelerated acquisitions. Three images with b=0 were acquired. For geometric
distortion correction, an additional image with b=0 was acquired with reversed
phase encoding direction. Other parameters were: FOV=220×220mm2, matrix=88×88,
resolution=2.5×2.5×2.5mm3, slices=56, TR/TE=4.9s/95ms,
BW/pixel=2104Hz, a GRAPPA factor=2. We calculated maps of WMTI metrics (axonal
water fraction [AWF], intra-axonal diffusivity [Daxon], extra-axonal
axial and radial diffusivities [De,a and De,r]), as well as DTI metrics (fractional anisotropy
[FA], mean, axial, radial diffusion coefficients [MD, AD, RD]) and DKI metrics
(mean, axial, radial kurtosis [MK, AK, RK]). Auditory-verbal working memory was
assessed using two WAIS-IV subtests: 1) Digit Span (DS) which includes DS
Forward [DSF], Backward [DSB] and Sequencing [DSS]; and 2) Letter-Number
Sequencing [LNS]. We performed tract-based spatial statistics (TBSS)6
with age and gender as covariates to reveal possible correlations between
working memory subtest scores and the diffusion metrics. The resulting statistical
maps from TBSS were thresholded at p < 0.05 (corrected for multiple
comparisons). Spearman’s partial rank correlation coefficients were also
calculated for those significant voxels on the skeleton, adjusted for age and
sex.RESULTS
In NCs, there were several brain regions demonstrating statistically significant positive correlation between AWF and LNS, the most complex of the set of four working memory tasks, most notably in parietal WM, left greater than right; others areas included right posterior limb of internal capsule, left body of corpus callosum, left posterior/superior corona radiata, bilateral superior longitudinal fasciculus, left inferior longitudinal fasciculus, bilateral anterior thalamic radiation and left cingulum (Fig.1). A small region in the right frontal WM (inferior fronto-occipital fasciculus) also showed a significant positive correlation between MK and LNS. For the MTBI group, no significant positive correlations were found from TBSS. No other diffusion metrics showed area of significant difference surviving correlation for multiple comparisons. Furthermore, no significant correlations were observed with the three relatively simpler digit span (DS) tasks. Fig.2 presents the scatter plots of each significant metric (AWF, MK) and LNS, showing that higher AWF (Fig.2(left); r=0.89, p < 0.001) and higher MK (Fig.2(right); r=0.92, p < 0.001) are associated with better performance on the LNS for those significant voxels on the skeleton for the NC group (black circle), but no significant correlations were observed for the MTBI group on the same location (red triangle).DISCUSSION
In this study, for the NC group, significant correlations were observed between AWF and LNS, and to a lesser degree between MK and LNS. Higher AWF has been previously shown in regions of higher axonal density and greater myelination both of which could contribute to greater efficiency in information processing.6 MK reflects degree of tissue microstructural complexity. Asymmetric correlation with left hemisphere microstructural measures is consistent with expected lateralization for tasks of auditory-verbal working memory tested in this case.7 The correlation in NCs was quite strong and highly statistically significant; however, none of these relationships persisted in the MTBI group. In MTBI, there is evidence of WM injury8 as well as altered patterns of brain activation after injury.9 These factors likely contribute to the loss of correlation between WM microstructure and performance on LNS task in MTBI subjects observed here.CONCLUSION
The relationship normally present in control subjects between measures of white matter microstructure and a complex task of auditory-verbal working memory is not observed in patients after MTBI and may reflect known WM injury and changes in functional organization that occur after injury.Acknowledgements
This work was supported in part by grant funding from the National Institutes of Health (NIH): R01 NS039135-11 and R21 NS090349, National Institute for Neurological Disorders and Stroke (NINDS). This work was also performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net), a NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4(10):829-839.
- Sandry, J. Working memory and memory loss in neurodegenerative disease. Neurodegener Dis Manag. 2015;5:1-4.
- Sandry, J., et al. Individual differences in working memory capacity predicts presponsiveness to memory rehabilitation after traumatic brain injury. Arch Phys Med Rehabil. 2016;97:1026-1029.
- Fieremans, E., et al. White matter characterization with diffusion kurtosis imaging. Neuroimage. 2011;58:177-188.
- Wechsler, D. Wechsler Adult Intelligence Scale. Fourth Edn. Pearson Assessment. 2008.
- Jelescu, I.O. et al. In vivo quantification of demyelination and recovery using compartment-specific diffusion MRI metrics validated by electron microscopy. Neuroimage. 2016;132:104-114.
- Thomason, M.E. et al. Development of spatial and verbal working memory capacity in the human brain. J Cogn Neurosci. 2009;21:316-332.
- Chung, S. et al. Characterizing white matter microstructural changes after mild traumatic brain injury based on diffusion white matter tract integrity (WMTI) and Shannon entropy. 25th ISMRM, Honolulu, Hi, USA. 2017;#4530.
- McAllister, T.W. et al. Brain activation during working memory 1 month after mild traumatic brain injury. Neurology. 1999;53:1300-8.
Figures
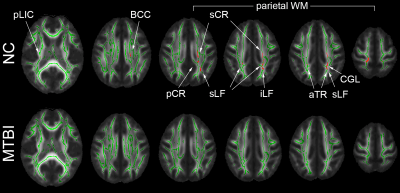
Figure
1. (Top) For the NC group, TBSS results show multiple areas of significant
positive correlation (corrected p<0.05) between AWF and LNS, particularly
involving left greater than right parietal WM. Other regions involved: right
posterior limb of internal capsule (pLIC), left body of corpus callosum (BCC),
left posterior/superior corona radiata (pCR/sCR), bilateral superior
longitudinal fasciculus (sLF), left inferior longitudinal fasciculus (iLF), bilateral
anterior thalamic radiation (aTR), and left cingulum (CGL). (Bottom) For the
MTBI group, no significant correlations were found.
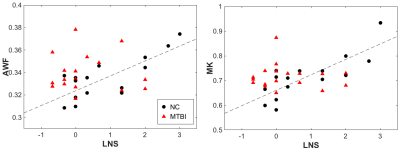
Figure
2. Scatter plots show strongly significant positive correlations (left) between
AWF and LNS (r = 0.89, p < 0.001) and (right) between MK and LNS (r = 0.92,
p <0.001) in normal controls (NC) (black circle). No such correlations were
found in the MTBI groups (red triangle).