4945
DTI Connectome Analysis in Moderate TBI: Parcellation Improves Detection of Relevant Injury and the Monitoring of Therapeutic Response1DUKE UNIVERSITY MEDICAL CENTER, DURHAM, NC, United States, 2RADIOLOGY, DUKE UNIVERSITY MEDICAL CENTER, DURHAM, NC, United States, 3NEUROLOGY, DUKE UNIVERSITY MEDICAL CENTER, DURHAM, NC, United States
Synopsis
Conventional DTI readouts such as fractional anisotropy (FA), axial diffusion (RD) and radial diffusion (RD) show distinct alterations following closed head injury but do not correlate well with measured functional and cognitive outcomes. 3D DTI with parcellated connectome analysis reveals neural fiber networks between brain regions that are most vulnerable to closed head (acceleration-deceleration) injury and also most relevant to measurable functional deficits. In this study, fiber tract number between hippocampus and cortex is the most sensitive marker of both motor and memory deficits and therapeutic improvement following administration of a novel neuroprotective therapeutic agent.
Introduction:
Non-hemorrhagic diffuse axonal injury (DAI) is increasingly recognized as an fundamental, long-range mechanism responsible for lasting neurological dysfunction following TBI1,2. DTI MRI has shown promise for detecting DAI, yet it remains uncertain which DTI parameters will be the most sensitive and reliable for clinical application. We have performed ex-vivo high-resolution DTI Tractography in a mouse model of TBI to probe the potential utility of different DTI biomarkers for detecting clinically relevant injury. In this model, DTI parameters such as regional FA, AD, and RD are altered following injury but do not correlate with vestibulomotor (rotorod) or memory (Morris Water Maze) dysfunction. We have found, however, that one scalar DTI parameter, fiber tract number (FTN), is significantly altered in several brain regions following TBI, and when parcellated specifically between hippocampus and neocortex, correlates strongly with functional outcomes and therapeutic response.Materials and Methods:
Experimental design: N= 17 mice (C57Cl/6J). Sham = 5, Vehicle= 6, Treated = 6. Treatment group was administered a novel quinone oxidoreductase II (QR2) inhibitor previously shown to have strong neuro-protective efficacy in TBI4. TBI was elicited using an established closed head injury model2 involving a single midline impact to exposed skull with spontaneous recovery. Mice were perfused at 6 weeks after injury. Brains were excised and stored in 0.5% ProHance-doped formalin3. Image Acquisition: Brains in Fomblin were scanned on a Bruker 7T scanner, quadrature volume-transmit and surface receive coils. The MRI protocol comprised: (1) 3D T1-weighted FLASH sequence with FOV = 1.8 cm X 1.8 cm X 1.8 cm; matrix = 256 x256 x 256; resolution = 70 x 70 x 70 μm/pixel; TE/TR = 6/30 ms; averages = 16; flip-angle = 34; scan time = 6 hrs 33 mins; (2) High resolution spin-echo based 3D DTI with FOV = 1.8 cm x 1.8 cm x 1.8cm; matrix = 128 x 128 x 128; resolution = 141 x 141 x 141 μm/pixel; TE/TR = 25/250 ms; diffusion directions = 60; Ao images = 5; B-value per direction = 1500 S/mm2 ; Post-Processing: Mouse brains were registered to a reference template from Wake forest University’s Mouse Database and segmented using ITK-SNAP. DTI parameters were calculated using TrackVis.Results and Discussion:
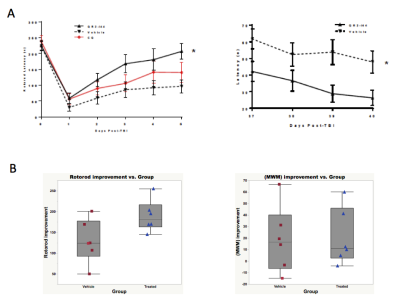
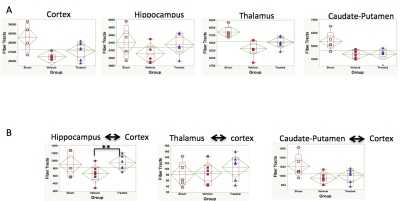
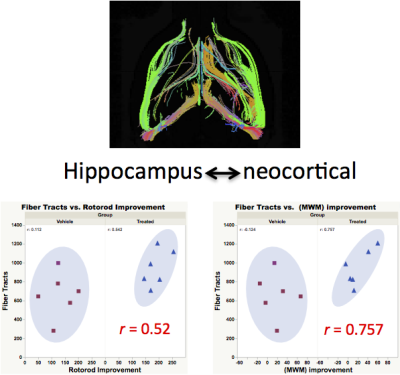
Figure 1 shows the functional therapeutic response in a mouse model of TBI following administration of novel 4-aminoquinoline therapeutic selective for the putative enzyme target quinone oxidoreductase II. Fig 1A shows the deficits and improvements in vestibulomotor function (rotorod, left) and consolidative memory (Morris water maze, right) following TBI and after therapy from the larger cohorts of the full study, whereas Fig 2B shows the motor and memory improvements in the sub-selection of animals from each cohort analyzed by MRI. In data not shown, FA and AD are uniformly decreased and RD increased in selected brain regions including cortex, hippocampus, basal ganglia, thalamus, and corpus collosum/external capsule. None of these DTI readouts, however, correlate with functional outcome, and none are improved with therapy. Fig 2A shows uniformly decreased FTN in different brain regions as well as a trend towards mitigation of FTN decrements in all brain regions except basal ganglia. When FTN is calculated after parcellation between specific brain regions (Fig 2B), there is statistically significant improvement in FTN in connections specifically between hippocampus and cortex, with trends in improvement also observed between thalamus and cortex and basal ganglia and cortex. In Fig 3, strong correlations between hippocampal-neocortical FTN and vestibulomotor (left) and memory (right) function are revealed following therapy. Strong correlations between function and un-parcellated FTN measurements of pass-through fibers passing through different brain regions were weak or non-existent, with the exception of neocortex (data not shown).Conclusions:
Our data suggest DTI connectome analysis of specific brain regions is a more sensitive and specific approach for detecting and monitoring clinically relevant brain injury following TBI. In a mouse model of closed head injury, FA, AD, and RD are all significantly altered within and between different brain regions, but none of these DTI readouts correlate with functional outcome or therapeutic response. 3D DTI measurements of FTN are altered within and between brain regions. FTN between hippocampus and neocortex following TBI correlates most strongly with both functional assessments and therapeutic response.Acknowledgements
No acknowledgement found.References
1. Bianchi A, Bhanu B, Obenaus A. Dynamic Low-Level Context for the Detection of Mild Traumatic Brain Injury. Ieee Transactions on Biomedical Engineering 2015;62:145-153. 2. Laskowitz DT, Song P, Wang H, et al. Traumatic Brain Injury Exacerbates Neurodegenerative Pathology: Improvement with an Apolipoprotein E-Based Therapeutic. Journal of Neurotrauma 2010;27:1983-1995.
3. Johnson GA, Calabrese E, Badea A, Paxinos G, Watson C. A multidimensional magnetic resonance histology atlas of the Wistar rat brain. Neuroimage 2012;62:1848-1856.
4. Venkatraman TN et al., Proc ISMRM 2017; 4527.
Figures