4943
Exploring the effects of concussion history on quantitative estimation of temporal delays and cerebrovascular reactivity: A novel approach combining hypercapnia and hyperoxia in collegiate football players1Centre for Neuroscience studies at Queen's University, Kingston, ON, Canada, 2Department of Radiology at the University Medical Center Utrecht, Utrecht, Netherlands, 3Department of Surgery at Queen's University, Kingston, ON, Canada
Synopsis
Recent evidence suggests that concussion history may be associated with long-term structural and functional changes in the brain. However, little is known about the effects of head injury on vascular reactivity. In this study, we combined hypercapnic and hyperoxic respiratory manipulations to explore the effects of concussion history on hemodynamic latencies and tissue cerebrovascular reactivity (CVR) in football players. We found that, although tissue CVR was not different between the groups (P>0.05), time delays to hypercapnia were shorter in subjects with a history of concussion (P<0.05), which may suggest some compensatory mechanism in the vasculature that persists beyond clinical recovery.
Introduction
An increasing number of studies have shown structural1,2 and functional3–5 alterations in athletes with a history of concussion, despite reporting no clinical symptoms. Although recent evidence suggests that concussive injuries may cause transient damage to the vasculature of the brain,6,7 little is known about the long-term effects of concussion on cerebrovascular reactivity (CVR).
The aim of our study was to investigate whether collegiate football players with a history of concussion (‘hx’ group) demonstrated differences in CVR, as compared to age-matched football athletes with no history of head injury (‘no hx’ group). We implement a novel approach combining hypercapnic (HC) and hyperoxic (HO) respiratory manipulations, and RIPTiDE (Rapid interpolation at progressive time delays) data analysis,8,9 to gain insight on temporal delays associated with blood flow re-distribution (under vaso-active hypercapnia),10 and/or individual differences in cerebrovascular morphology.11
Methods
25 collegiate football athletes were recruited to participate in this study (meanage 20±1 years). All subjects were scanned at pre-season baseline within one month prior to participation in full contact practices. Of these 25 subjects, 15 reported having sustained at least one (N = 10) or two (N = 5) concussion throughout their career. Time post-injury ranged between 2-10 years (median = 4 years).
All subjects completed two 6 minutes boxcar respiratory manipulations (one HC targeted at +10 mmHg, and one HO targeted at +300 mmHg ), applied using an automated gas blending system (RespirActTM, Thornhill Research Inc., Toronto, ON). BOLD and CBF data were acquired on a Siemens 3.0T scanner using a 32-channel receiver head coil, and a dual-echo pseudo-continuous arterial spin labeling sequence (pCASL; TR = 4000 ms, TE1/TE2 = 10/30 ms, field of view = 250 x 250 mm, voxel size = 3.9 mm isotropic, post-labeling delay = 1000 ms, tagging duration 1.665s).12,13
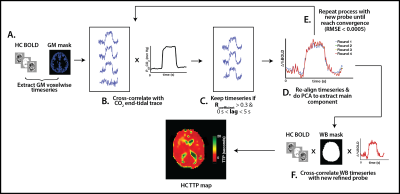
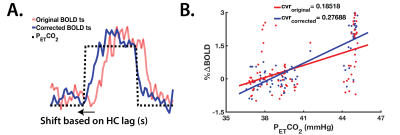
The RIPTiDE method (Figure 1) was implemented to isolate the hemodynamic component of the BOLD response, and characterize temporal delays during both HC and HO. HC temporal delays were also used to correct CVR (Figure 2) and estimate the maximal CVR, independent of the vascular response speed.
All parameters were compared between the groups using a multivariate analysis of variance (MANOVA), and 0.05 as the threshold for statistical significance. Outlier values were removed from each group prior to statistical analysis using Tukey’s method (G factor = 1.5).
Results and discussion
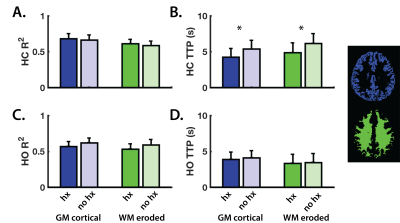
The RIPTiDE method generates two parameter maps: the squared correlation coefficient (R2) and the time to peak (TTP; seconds) maps.10 The R2 map reflects the proportion of the BOLD signal change that is modulated by the changes in blood CO2 (HC) and O2 (HO). Grey- and white-matter (GM;WM) R2 values for each challenge (Figure 3A and 3C) were not different between the groups (P>0.05), indicating that all subjects experienced similar proportional changes in BOLD signal following increases in arterial gaseous content.
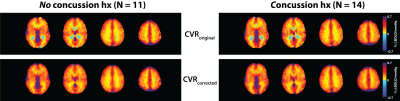
When comparing tissue HC-TTP, players with a history of concussion (‘hx’) showed significantly shorter delay times in both GM (P<0.05) and WM (P<0.05) (Figure 3B), although no differences in GM or WM CVRoriginal and CVRcorrected (P>0.05) were found between the groups (Figure 4). Differences in HC delay times may be related to the vaso-active nature of the HC stimulus, which can introduce temporal delays due to transient episodes of blood flow re-distribution between regions with different vascular reserve capacity.14 CVR did not differ between groups, suggesting factors such as longer arterial transit time relating to morphological differences in vasculature architecture, as contributors to longer temporal HC delays.15 Such elements are independent of CVR.
To test this idea, we estimated HO delays as an indirect measure of blood arrival time (and plasma-rich O2 as an endogenous contrast agent),16 to investigate whether longer HC delay times were due to transit time of the gas stimulus. Since HO is non-vasoactive,17,18 changes in BOLD contrast upon increases in arterial O2 content are independent of differences in the vascular tone, and thus, uncoupled from CVR.
HO temporal delays were not significantly different between the groups (P>0.05; Figure 3D). We speculate that shorter delays in the concussion history group resulted from adaptive mechanisms in the brain that follow the neurophysiological stresses resulting from concussion,19–21 which may persists beyond recovery from clinical symptoms. Our findings provide a novel perspective on the importance of considering multiple components of the BOLD contrast, along with concussion history, when exploring hemodynamic parameters of the brain in athletes.
Acknowledgements
The authors would like to thank Mr. Don Brien for his help with data collection.References
1. Churchill, N. W., Caverzasi, E., Graham, S. J., Hutchison, M. G. & Schweizer, T. A. White matter microstructure in athletes with a history of concussion: Comparing diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). Hum. Brain Mapp. 38, 4201–4211 (2017).
2. Churchill, N. et al. Brain structure and function associated with a history of sport concussion: a multi-modal MRI study. J. Neurotrauma 1–29 (2016). doi:10.1089/neu.2016.4531
3. Guskiewicz, K. M. et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 57, 719-726-726 (2005).
4. Ford, J. H., Giovanello, K. S. & Guskiewicz, K. M. Episodic memory in former professional football players with a history of concussion: an event-related functional neuroimaging study. J. Neurotrauma 30, 1683–701 (2013).
5. Churchill, N., Hutchison, M. G., Leung, G., Graham, S. & Schweizer, T. A. Changes in functional connectivity of the brain associated with a history of sport concussion: A preliminary investigation. Brain Inj. 31, 39–48 (2017).
6. Len, T. K. et al. Cerebrovascular reactivity impairment after sport-induced concussion. Med. Sci. Sports Exerc. 43, 2241–8 (2011).
7. Mutch, W. Alan C. Ellis, Michael J. Ryner, Lawrence N. Graham, Ruth. Dufault, Brenden. Gregson, Brian. Hall, Thomas. Bunge, Martin. Essig, M. Brain magnetic resonance imaging CO2 stress testing in adolescent post-concussion syndrome: pCASL findings. J. Neurosurg. (2015). doi:10.3171/2015.6.JNS15972.
8. Tong, Y., Bergethon, P. R. & Frederick, B. deB. An improved method for mapping cerebrovascular reserve using concurrent fMRI and near-infrared spectroscopy with Regressor Interpolation at Progressive Time Delays (RIPTiDe). Neuroimage 56, 2047–2057 (2011).
9. Frederick, B. deB, Nickerson, L. D. & Tong, Y. Physiological denoising of BOLD fMRI data using Regressor Interpolation at Progressive Time Delays (RIPTiDe) processing of concurrent fMRI and near-infrared spectroscopy (NIRS). Neuroimage 60, 1913–1923 (2012).
10. Donahue, M. J. et al. Time delay processing of hypercapnic fMRI allows quantitative parameterization of cerebrovascular reactivity and blood flow delays. J. Cereb. Blood Flow Metab. 36, 1767–79 (2016).
11. Donahue, M. J. et al. Bolus arrival time and cerebral blood flow responses to hypercarbia. J. Cereb. Blood Flow Metab. 34, 1243–52 (2014).
12. Alsop, D. C. et al. Recommended implementation of arterial spin-labeled Perfusion mri for clinical applications: A consensus of the ISMRM Perfusion Study group and the European consortium for ASL in dementia. Magn. Reson. Med. 73, 102–116 (2015).
13. Wu, W., Buxton, R. B. & Wong, E. C. Vascular space occupancy weighted imaging with control of residual blood signal and higher contrast-to-noise ratio. IEEE Trans. Med. Imaging 26, 1319–27 (2007).
14. Bhogal, A. A. et al. Examining the regional and cerebral depth-dependent BOLD cerebrovascular reactivity response at 7T. Neuroimage 114, 239–248 (2015).
15. Blockley, N. P., Driver, I. D., Francis, S. T., Fisher, J. A. & Gowland, P. A. An improved method for acquiring cerebrovascular reactivity maps. Magn. Reson. Med. 65, 1278–86 (2011).
16. Liu, P. et al. Multiparametric imaging of brain hemodynamics and function using gas-inhalation MRI. Neuroimage 146, 715–723 (2017).
17. Bulte, D. P., Chiarelli, P. a, Wise, R. G. & Jezzard, P. Cerebral perfusion response to hyperoxia. J. Cereb. Blood Flow Metab. 27, 69–75 (2007).
18. Bulte, D., Chiarelli, P., Wise, R. & Jezzard, P. Measurement of cerebral blood volume in humans using hyperoxic MRI contrast. J. Magn. Reson. Imaging 26, 894–899 (2007).
19. Shaw, N. a. Neurophysiology of concussion. Prog Neurobiol 67, 281–344 (2002).
20. Giza, C. C. & Hovda, D. a. The Neurometabolic Cascade of Concussion. J. Athl. Train. 36, 228–235 (2001).
21. Choe,
M. C. The Pathophysiology of Concussion. Current Pain and Headache Reports
20, (2016).
Figures