4935
Assessment of Intravenous Immunoglobulin treatment on BBB function following inflammation1School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin, Dublin, Ireland, 2School of Biochemistry and Immunology, Trinity College Dublin, Dublin, Ireland
Synopsis
The blood brain barrier (BBB) is a complex structure that separates brain interstitial fluid from blood. BBB dysfunction is a key feature of Central Nervous System diseases. This in vivo study examines the effects of Intravenous Immunoglobulin (IVIg), regularly used to treat immune and inflammatory diseases, on BBB function following LPS-induced BBB disruption. The change in permeability of the BBB was evaluated by MRI, using gadolinium. Our results suggest that LPS breach the integrity of the BBB with regional specificity and can be reversed by IVIg administration.
Introduction
The blood-brain barrier (BBB) forms a protective barrier, with the important function of maintaining brain homeostasis. Neuro-inflammation disrupts BBB integrity either during initiation and/or progression of many neurological diseases such as, Parkinson’s disease, multiple sclerosis, Alzheimer’s disease and schizophrenia1. While BBB disruption contributes to the pathogenesis of many CNS diseases, it may be that the disease-induced alterations seen in BBB permeability may affect the treatment of these diseases2. Intravenous immunoglobulin (IVIg) is used to treat immune and inflammatory diseases3. It is becoming a widely used treatment option for peripheral neuropathologies, such as Guillain-Barré syndrome. Since neuro-inflammation is known to cause BBB disruption, the potential of IVIg as a treatment for neurological diseases needs further investigation. The goal of the study was to investigate the effect of IVIg in BBB disruption. Lipopolysaccaride (LPS) is a potent toxin and plays a role in the initiation of the inflammatory cascade. It causes a disruption in the BBB by binding to toll-like receptor 4 receptors on the surface of endothelial cells4. The change in permeability of the BBB was evaluated by MRI using gadolinium as a contrast agent.
Method
The experimental protocols employed in this study were approved by the local Animal Research Ethics Committee and HPRA (Ireland), in accordance with the EU Directive (2010/63/EU). The mice were categorized into 4 groups: Mice were injected with saline (n=6) or 1mg/kg LPS (n=4) i.p. 3 hours prior to MRI scanning or mice were injected with saline (n=3) or 1mg/kg LPS (n=6) i.p. 3 hours prior to scanning and then with 1.5g/kg IVIg one hour post administration of LPS. BBB integrity was assessed in vivo using a dedicated small rodent 7T MRI Bruker system. Images of the brain were visualised using T1 weighted 2D MRI sequence before and following administration of 100µl of a 170 mmol solution of Magnevist administered via a tail vein catheter. The parameters for this T1 flash sequence were; average: 10, repetition: 1, echo time: 7ms, repetition time: 4443.985 ms, scan time: 18 min 56 sec 600 ms, flip angle: 30 degrees, slices: 25, slice orientation: axial, read orientation: Le-Rt, slice thickness: 0.250mm, matrix: 384x384, field of view: 20x20mm2, pixel resolution: 52 µm/pixel. Coronal orientated slices for pre-injection and for post-injection of gadolinium from each mouse were saved and analysed with freeware medical image analysis software, MIPAV. Regions of interests (ROIs) were selected for each image depending on the structures present in that particular slice. Seven structures of the mouse brain were studied including the cortical regions: Signal to Noise Ratio (SNR) and percentage difference SNR were calculated using the equation: SNR (%) = [(post Gd – pre-Gd)/(pre-Gd)]*100. Statistical significance was assessed by a one-way Anova and Tukey’s post hoc test. A value of P < 0.05 was considered statistically significant.Results
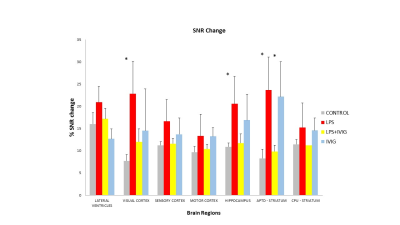
A single dose of LPS 1mg/kg was chosen because it has been shown to increase the permeability of the BBB 1 hour after administration in mice5. The MRI scans were taken 3 hours post-injection of LPS, so it is possible that the permeability of the BBB peaked after one hour and had begun its repair to the natural state by the time the MRI scans were taken. LPS does not cause a disruption to the BBB in every regions of the mouse brain. No significant difference in SNR can be seen between the control group and LPS 1mg/kg group for the lateral ventricles, sensory cortex, motor cortex and caudate Putamen. However, there is a significant difference for the visual cortex, hippocampus and APTD (striatum). In these 3 regions, the administration of 1.5g/kg of IVIg has reduced the permeability of the BBB to gadolinium in our conditions.Discussion and Conclusion
A dose of 1mg/kg LPS in mice causes an increase in the BBB permeability in the visual cortex, hippocampus and APTD (striatum). However, the BBB is relatively resistant to LPS-induced disruption in the lateral ventricle, sensory cortex, motor cortex and caudate putamen. Furthermore, 1.5 g/kg of IVIg has partially restored the integrity of the BBB that was compromised with the administration of LPS. These results suggest that LPS breaches the integrity of the BBB with regional specificity. Given that LPS can disrupt the BBB in the visual cortex and APTD, it is worth investigating in future experiments if a higher dose of LPS will disrupt the BBB in the other regions. It is also worth investigating if the use of a smaller contrasting agent such as manganese will allow for better visualisation of BBB disruption in these resistant LPS-induced disruption regions.Acknowledgements
No acknowledgement found.References
1- Stolp et al. Neuropathol Appl Neurobiol. 2009 Apr;35(2):132-46.
2- Lochhead et al. AAPS journal, 2017;19(4):910-20.
3- Misra et al. J Neurol. 2005;252 Suppl 1:I1-6.
4- Diamond et al. Nat Rev Immunol. 2009;9(6):449-56.
5- Jungula et al. Neurosci Lett. 2013 Sep 13;551:23-7.
Figures