4932
Altered cerebral blood flow before and after 4-weeks of neurostimulation in patients with episodic migraineLars Michels1, Jessica Aschmann2, Franz Riederer1, Andreas Gantenbein1,3, Roger Luechinger4, Spyros Kolllias1, and Peter Sandor3
1University Hospital Zurich, Zurich, Switzerland, 2Unversity of Zurich, Zurich, Switzerland, 3Rehaclinic Bad Zurzach, Zurzach, Switzerland, 4Swiss Federal Institute of Technology and the University of Zurich, Zurich, Switzerland
Synopsis
Cerebral blood flow is altered in migraineurs but it is unknown if this can be changed by repetitive neurostimulation, leading to less migraine attacks. In a double blind and sham-controlled study, we used arterial spin labeling MRI and transcranial direct current stimulation (tDCS) to address this question. Four weeks of real tDCS diminished initial hyperperfusion in patients with episodic migraine in pain processing brain regions (p < 0.001). In addition, less migraine attacks occurred after real tDCS compared to baseline (p < 0.05). Our results indicate a regulating effect of tDCS on cerebral blood flow and clinical outcome in migraineurs.
Introduction
Based on the literature 1, 2, altered cerebral blood flow (CBF) has been observed during migraine attacks. Yet, it is unknown abnormal if CBF can be modulated by non-invasive brain stimulation. Transcranial direct current stimulation (tDCS) has been used in patients with episodic migraine (EM) and resulted in a reduction of migraine days compared to baseline3. Yet, this study was not sham controlled and did not examine the associated changes in the brain, including CBF. We hypothesized that CBF will be different between sham and repetitive (4 weeks) real tDCS and that this neuronal reorganization process should be paralleled by a clinical improvement of the primary outcome variable (number of migraine days).Methods
We examined 17 adult patients with EM, which already underwent clinical examination and ASL-MRI at two time points (baseline, 1. follow-up). We recorded attack frequency, pain intensity, aura occurrence and medication. To assess CBF (during the interictal period), we used non-invasive arterial spin labeling magnetic resonance imaging (ASL-MRI) at three time points (baseline, maximally 4 weeks after tDCS, and 6 months after baseline). Specifically, CBF was recorded with a 2D pseudo-continuous ASL (pCASL) sequence4 with background suppression (repetition time (TR)/echo time (TE) = 4200/16 ms, flip angle: 90°, voxel size: 3 x 3 mm, 20 slices, thickness: 6 mm, no gap, labeling duration: 1.65 s, post-labeling delay: 1.53 s) implemented on a Philips 3 Tesla Ingenia scanner. Equilibrium brain tissue magnetization (M0) images (30s) were recorded to correct for proton density. The ASL analysis was performed with the ASL toolbox5. Pre-processing included motion correction, spatial smoothing (6 mm in plane) and normalization to the MNI template brain. CBF quantification was done using the one-compartment model 6. CBF difference images were achieved by simple subtraction to minimize spurious BOLD contaminations within the CBF signal7. We calculated planned contrasts (t-tests, p < .01, corrected) for within-group differences related to scan time point (baseline and follow-up) and between-group differences (sham versus real tDCS). For real tDCS, anodal tDCS (1 mA) was applied over the occipital cortex (occipital protuberance) for 4 weeks (1200 seconds/day) at home. Duration of the “treatment” interval was identical for sham tDCS but patients only received a low (< 0.1 mA) current, with initial 8s fade-in phase, 30s 1mA tDCS, and 5s fade-out phase, to elicit the same sensations on the skin. Prior to the study, we verified that the current flow was maximal at the occipital protuberance by simulation experiments (cathode was placed to the vertex).Results
Eight EM received real tDCS and nine EM received sham stimulation for 4 weeks and already underwent baseline and the first follow-up ASL-MRI. Patients did not differ with respect to sex, age, handedness, and migraine attacks at baseline (all p > .05). None of the patients stopped the stimulation, had a migraine attack during stimulation, or felt disturbed by the stimulation. Week tingling sensation was noticed by some patients for both sham and real tDCS. Real tDCS lead to a reduction in migraine days (from 9 to 8.2 (1. follow-up) and 6.2* (2. follow-up, *p < 0.05 comparing to baseline), whereas sham stimulation lead to a non-significant increase in migraine days (from 6.7 days to 8 days and 8.5 days, respectively). Two of the EM were migraine-free 6 months after baseline. For real tDCS, CBF was higher at baseline compared to the 1. follow-up in pain processing brain regions (e.g. insula, medial prefrontal cortex, thalamus). None brain regions showed higher CBF at 1. follow-up compared to baseline. Comparing sham to real tDCS (at 1. follow-up), revealed higher CBF for sham tDCS in the medial prefrontal cortex, subgenual anterior cingulate cortex, somatosensory cortex, insula, superior parietal lobe, and visual cortex (cuneus).Discussion
Our results indicate altered CBF in the untreated group of EM patients. This was also paralleled by the absence of any longitudinal reduction in migraine days. Yet, in line to a previous study, real tDCS significantly reduces migraine days in EM treated with real tDCS3. A novel finding was that real tDCS lowers CBF (compared to baseline and sham, respectively) in brain regions associated with pain processing, such as the insula and prefrontal cortex. In addition, brain regions linked to cognitive control (e.g. superior parietal lobe)8, 9 showed lower CBF during real tDCS versus sham tDCS.Conclusion
Non-invasive tDCS can alleviate migraine attack frequency and reduces CBF in areas involved in pain processing and attentional control.Acknowledgements
We thank all patients for their participation. We thank Drs. Ruff, Schoenen, and Moisa for their constructive feedback on the study design.References
1. Pollock, J.M., et al. Migraine associated cerebral hyperperfusion with arterial spin-labeled MR imaging. AJNR. American journal of neuroradiology 29, 1494-1497 (2008). 2. May, A. New insights into headache: an update on functional and structural imaging findings. Nature reviews. Neurology 5, 199-209 (2009). 3. Vigano, A., et al. Transcranial Direct Current Stimulation (tDCS) of the visual cortex: a proof-of-concept study based on interictal electrophysiological abnormalities in migraine. The journal of headache and pain 14, 23 (2013). 4. Wong, E.C., Buxton, R.B. & Frank, L.R. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR in biomedicine 10, 237-249 (1997). 5. Wang, Z., et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging 26, 261-269 (2008). 6. Buxton, R.B., et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 40, 383-396 (1998). 7. Liu, T.T. & Wong, E.C. A signal processing model for arterial spin labeling functional MRI. Neuroimage 24, 207-215 (2005). 8. Shomstein, S. Cognitive functions of the posterior parietal cortex: top-down and bottom-up attentional control. Front Integr Neurosci 6, 38 (2012). 9. Shomstein, S., Kravitz, D.J. & Behrmann, M. Attentional control: temporal relationships within the fronto-parietal network. Neuropsychologia 50, 1202-1210 (2012).Figures
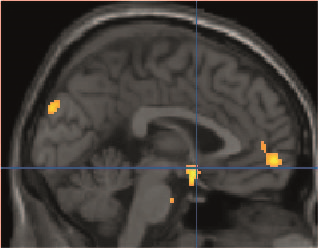
Figure 1. Summary of ASL-MRI
results comparing sham and real tDCS. Patients with EM demonstrated hyperperfusion
during sham tDCS (compared to real tDCS) in several brain regions (e.g., cuneus,
medial prefrontal cortex, and subgenual anterior cingulate cortex (blue
cross). Results are shown at a voxel-threshold of p < 0.01 (corrected). EM: episodic migraine, tDCS: transcranial
direct current stimulation.
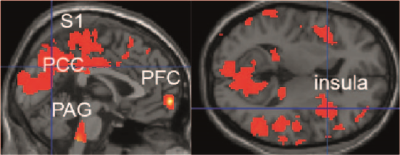
Summary of effects of real tDCS on CBF comparing
baseline and 1. follow-up ASL-MRI in EM. Hyperperfusion was seen at baseline in
pain processing regions. Results are shown at a voxel-threshold of p < 0.01 (corrected). EM: episodic
migraine, tDCS: transcranial direct current stimulation, S2: secondary
somatosensory cortex, PAG: periaqueductal grey, PCC: posterior cingulate cortex,
PFC: prefrontal cortex.