4926
Increased vascular permeability in the lenticulostriate arteries results in increased hemosiderin deposition in the basal ganglia in aging and cognitive impairmentAxel Montagne1, Giuseppe Barisano2, Meng Law1,2,3, Farshid Sepherband3, Arthur Toga3, and Berislav Zlokovic1
1Zilkha Neurogenetics Institute, University of Southern California, Los Angeles, CA, United States, 2Radiology, University of Southern California, Los Angeles, CA, United States, 3Stevens Institute of Neuroimaging and Informatics, University of Southern California, Los Angeles, CA, United States
Synopsis
The intramural periarterial drainage pathway is critical for the elimination of metabolic waste products from the brain. In a number of neurological diseases such as Alzheimer’s Disease, blood-brain barrier damage and increased vascular permeability may play an important role in the pathogenesis. Leakiness of the blood-brain barrier allows fibrin(ogen), hemosiderin and metabolic wastes to exudate and deposit around the vessels in the basal ganglia. In order to test this hypothesis, we evaluated the relationship between blood-brain barrier permeability measured as ktrans and hemosiderin deposition in 76 subjects scanned at 3T MRI.
Introduction
In Alzheimer’s disease (AD), cerebral interstitial fluid (ISF) and amyloid-β (Aβ) are eliminated from the brain along membranes of capillaries and arteries, the intramural periarterial drainage (IPAD) pathway1. With advancing age and arteriosclerosis (i.e., the stiffness of arterial walls) this pathway fails in its function and Aβ accumulates in the walls of arteries. With decreasing cerebral blood flow (CBF), increasing blood-brain barrier (BBB) permeability, decreasing cerebrospinal fluid (CSF) and ISF clearance of Aβ and other metabolic wastes, there is increased deposition of fibrin(ogen), hemosiderin and metabolic wastes in and around the lenticulostriate arteries. Even less is known of the significance of the perivascular spaces and brain lymphatics, the existence of which is somewhat controversial2,3. Some believe the CSF and ISF is cleared via these IPAD pathways into the cervical lymph nodes1,4,5. However, it is likely that the CSF and ISF have important roles as the “garbage” clearance for the brain6. There is no question that in a number of neurological diseases such as AD, BBB damage and increased vascular permeability, together with pathological changes in CBF, ISF and CSF flow or circulation may be synergistically involved in the pathogenesis7. We hypothesize that in aging and perhaps even more so in AD, an increase in the BBB permeability measured with Ktrans should correlate with increased hemosiderin deposition in the basal ganglia.Methods
We studied 76 subjects with vascular risk factors from the Alzheimer’s Disease Research Center at University of Southern California (USC). Young and old healthy subjects were also used as controls. MRI scans were performed on both a GE HDxT 3T and Siemens Prisma 3T at the Center for Image Acquisition, Institute of Neuroimaging and Informatics at USC. In all 76 subjects, we quantified the degree of hemosiderin deposition in the basal ganglia using a 3-point scale from the high resolution SWI. In order to assess BBB permeability, dynamic contrast-enhanced (DCE)-MRI was performed using a 3D fast low angle shot (FLASH) sequence and an intravenous bolus of Gd-DTPA (0.05 mmol/kg) administered at 20 sec after the beginning of the DCE scan (TR/TE = 7.3/2.3ms; flip angle = 30°; matrix = 256x192x24; section thickness = 5 mm; rate-2 GRAPPA, and ~15 sec per volume, 15 min scan time). Post-processing of DCE-MRI data will yield maps of BBB permeability (Ktrans) of Gd-DTPA and vascular volume as outlined in our publications8–10.Results
We found that patients which demonstrated higher vascular permeability Ktrans demonstrated greater hemosiderin deposition in the basal ganglia including putamen and globus pallidus areas. We utilized a linear regression and the Pearson’s Chi-squared test and found a correlation between the Ktrans within the globus pallidus/putamen and the hemosiderin deposition in the brain (p < 0.001).Conclusions
Pre-clinical and human studies have shown that during normal aging there is leakiness of the BBB and decrease in blood flow to the brain. This disruption to the BBB in the white matter microcirculation causes accumulation of toxic blood-derived fibrin(ogen) deposits, hemosiderin and other blood products within the vessel wall, perivascular spaces and adjacent white matter. This BBB breakdown may lead to white matter microstructural changes and eventually axonal and neuronal loss. These are likely the underlying mechanisms seen in vascular cognitive impairment and some of the vascular contributions to AD.Acknowledgements
This study was supported by the US National Institutes of Health grant UH2NS100614 and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health Award Number P41EB015922. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. #ML partially funded by NIH/NIA P50-AG05142, NIH P01AG052350, NIH P01AD06572.References
- Diem AK, MacGregor Sharp M, Gatherer M, Bressloff NW, Carare RO, Richardson G. Arterial Pulsations cannot Drive Intramural Periarterial Drainage: Significance for Aβ Drainage. Front Neurosci. 2017;11(August):1-9. doi:10.3389/fnins.2017.00475.
- Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. doi:10.1126/scitranslmed.3003748.
- Smith AJ, Yao X, Dix JA, Jin B-J, Verkman AS. Test of the “glymphatic” hypothesis demonstrates diffusive and aquaporin-4- independent solute transport in rodent brain parenchyma.
- Bakker ENTP, Bacskai BJ, Arbel-Ornath M, et al. Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cell Mol Neurobiol. 2016;36(2):181-194. doi:10.1007/s10571-015-0273-8.
- Carare RO, Bernardes-Silva M, Newman TA, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34(2):131-144. doi:10.1111/j.1365-2990.2007.00926.x.
- Nedergaard M. Garbage truck of the brain. Science (80- ). 2013;340(6140):1529-1530. doi:10.1126/science.1240514.
- Marchi N, Banjara M, Janigro D. Blood–brain barrier, bulk flow, and interstitial clearance in epilepsy. J Neurosci Methods. 2016;34(5):352-359. doi:10.1177/0963721414541462.Self-Control.
- Barnes SR, Ng TSC, Santa-Maria N, Montagne A, Zlokovic B V, Jacobs RE. ROCKETSHIP: a flexible and modular software tool for the planning, processing and analysis of dynamic MRI studies. BMC Med Imaging. 2015;15:19. doi:10.1186/s12880-015-0062-3.
- Barnes SR, Ng TSC, Montagne A, Law M, Zlokovic B V., Jacobs RE. Optimal acquisition and modeling parameters for accurate assessment of low K-trans blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magn Reson Med. 2016;75(5):1967-1977. doi:10.1002/mrm.25793.
- Montagne A, Barnes SR, Sweeney MD, et al. Blood-Brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296-302. doi:10.1016/j.neuron.2014.12.032.
Figures
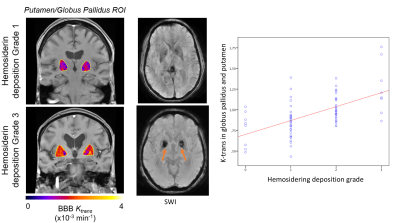
Figure 1: DCE-MRI Ktrans and SWI at 3T demonstrate a correlation between the vascular
permeability and the amount of hemosiderin deposition in the basal ganglia. Linear
regression and Pearson’s Chi-squared test demonstrate a significant correlation
between the Ktrans and the hemosiderin deposition. This is compatible with our
hypothesis that vascular dysfunction and leakiness of the BBB allows fibrin(ogen)
and hemosiderin to exude from the lenticulostriate arteries into the
perivascular spaces and adjacent white matter. In turn this will result in
damage to the oligodendrocytes and myelin ultimately causing white matter
hyperintensity in patients with vascular cognitive impairment.