4925
Glycine is an early 'biomarker' of blood brain barrier breakdown in Gliomas1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Department of Neurological Surgery, UT Southwestern Medical Center, Dallas, TX, United States, 3Department of Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 4Department of Neurology and Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, United States, 5Harold C. Simmons Cancer Center, UT Southwestern Medical Center, Dallas, TX, United States, 6Department of Internal Medicine, UT Southwestern Medical Center, Dallas, TX, United States, 7Annette Strauss Center for Neuro-Oncology, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Cancer cells may use altered metabolic pathways with respect to their normal counterparts; this metabolic switch is necessary to support their rapid proliferation in oxygen- and nutrient-poor conditions. A few of the metabolites are up-regulated while some others are down-regulated or unaltered. High grade malignant tumors present with enhancement on T1-weighted (T1w) image. Enhancement on post-contrast T1w images is an indicative of breakdown of blood brain barrier (BBB). The increased number of tumor cells may stress the vessels and, thus BBB tend to rupture. A non-invasive bio-marker that can predict the disruption of BBB will be of great clinical significance for assessing the tumor aggressiveness. Here, we show elevated Gly can be a potential bio-marker for predicting the tumor’s potential to present with ruptured BBB.
Introduction
Tumors reprogram their metabolism to support their rapid cell growth, resulting in altered metabolic profiles. Recent metabolic studies have reported a key role of glycine (Gly) in supporting rapid cellular proliferation (1,2). Up-regulation of Gly synthesis enzymes are found to correlate with greater mortality and worse prognosis in breast cancer (3). Abnormal level of Gly in brain tumors have been reported in prior magnetic resonance spectroscopy (MRS) studies (4,5,6). Highly aggressive tumors may cause blood vessels to rupture and leak as a consequence of increased stress from the uncontrollably proliferating cells. Enhancement on post-contrast T1-weighted (T1w) images indicate an abnormality of the BBB with extravasation of the contrast molecules in aggressive gliomas. Here, we have measured Gly level in glioblastoma patients using a triple-refocusing 1H MRS sequence in vivo (7). Gly elevation was evaluated with clinical features that identify broken BBB, such as enhancement on T1w post-contrast images. In this study we have identified Gly as an early metabolic bio-marker that predicts damage to BBB. A metabolic bio-marker predictive of damage to BBB will help to identify tumors with malignant property.Methods
A Triple-refocusing-sequence tailored for Gly detection, as described previously (7) was used for in-vivo 1H MRS measurements in 5 Glioblastoma (Grade IV) patients on a whole-body 3T research scanner (Philips Medical Systems). Spectra were obtained from voxels in tumors identified by T2-weighted fluid-attenuated-inversion recovery (T2w-FLAIR) imaging. The T1w post-contrast images were obtained on the scanners at the clinical site. The MRS voxel-size was 3-5 mL depending on the tumor-volume. MRS acquisition parameters included TR=2s, sweep width=2.5 kHz, sampling-points=2048, and signal-averages 128-256 depending on the voxel-size, with vendor-supplied water-suppression scheme. Unsuppressed water was obtained with short-TE (14 ms) STEAM and TR=20s as a reference in metabolite quantification. Spectral-fitting was performed with LCModel-software using numerically-calculated basis-spectra of 22 metabolites. Metabolite concentrations were calculated by setting the mean total-Creatine estimate of the medial occipital brain from healthy subjects at 8 mM.Results and Discussions
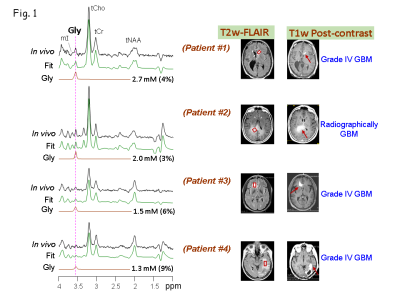
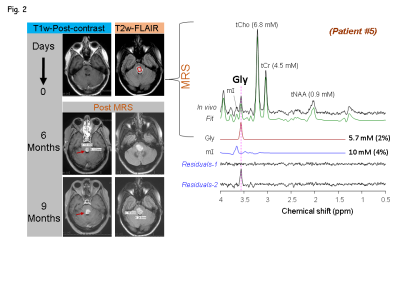
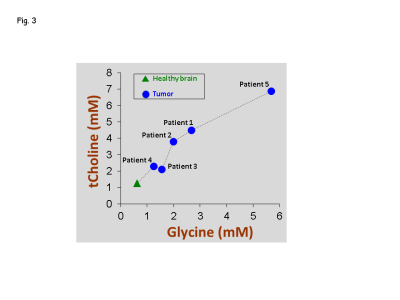
Of the five patients, three (Patients # 1, 3 and 4) had biopsy-proven glioblastoma (grade IV GBM) (Fig.1), and other two patients (Patients # 2 and 5) had radio-graphically suggested malignant medio-occipital (Fig.1) and brainstem gliomas (Fig.2), respectively. Gly signal at 3.55-ppm was visually discernible in the data from all the patients. All the 4 tumor patients showed ~2 - 5 fold elevated Gly level (Fig.1) compared to the normal levels of Gly (7) (0.6 mM in pure gray-matter regions and 0.3 mM in pure white-matter rich region). Noticeably, all the tumor locations that presented with high Gly were found to be enhancing on the T1w post-contrast images suggestive of broken BBB in these tumors. However, another patient (Patient #5) that presented with a brainstem lesion on FLAIR images without any enhancement on T1w images, showed ~6 mM of Gly (Fig.2), which was ~15 fold higher than the normal levels of Gly in a healthy brainstem (0.37 mM (7)).Given the extensive elevation of Gly in this patient, and the surgical challenges in the brainstem tumor, the radiological interpretation was then made as a grade IV glioma and a decision was made to treat the patient with chemo-radiation without biopsy. Noticeably, after 6 months of our finding of elevated Gly from the MRS scan, enhancement was observed in the brain-stem lesion on the post-contrast T1w images. Patient could not be brought for research MRS scans at this time as the patient was in hospice care due to extreme clinical-complications. However, the enhancement progressed over time, thus ruling out the possibility of pseudo-enhancement caused by treatments. Similar to the other 4 tumors, elevated Gly in the brain stem tumor also presented with enhancement, although a few months after our MRS finding. Elevations in Gly-level in all the five tumors was found to positively correlate (R2 =0.96, p=0.001) with elevations in choline level (Fig.3).Conclusion
Elevated level of Gly in tumors with enhancement on post-contrast images, suggest that Gly -elevation measurements may help identify aggressively growing tumors that have malignant properties. Presence of elevated Gly in the brain-stem tumor patient prior to the observable enhancement suggests that tumors may present with elevated Gly prior to the manifestation of BBB breakdown. Pseudo enhancements/pseudo-progressions in tumors, which are often observed after surgical interventions or radiation treatments, can be delineated by monitoring the Gly-level in the enhancing region. High-Gly level may provide surplus one carbon unit needed for nucleotide biosynthesis which will lead to rapid cellular proliferation. As a consequence of enhanced Gly-metabolism in tumors, cellularity of the tumors may increase as indicated by the increased choline level in our study.Acknowledgements
We thank Ms. Kelley Derner, and Lucy Christie for study coordination. This study was supported by National Cancer Institute of the National Institutes of Health under Award Number R01CA184584 and by a Cancer Prevention Research Institute of Texas grant RP130427.References
1. Jain et al. metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 2012;336:1040-1044.
2. Tomita et al. Cancer Systems biology, metabolomics, and cancer metabolism. Science 2012;336:990-99.
3. Zhang et al. Prognostic and therapeutic value of mitochondrial serine hydroxyl-methyl transferase-2 as a breast cancer biomarker. Oncol Rep. 2016;36:2489-2500.
4. Prescot et al. In vivo detetction of brain glycine with echo-time-averages 1H Magnetic Resonance Spectroscopy at 4.0T. Magn Res Med. 2006;55:681-686.
5. Ganji et al. In vivo 1H MRSI of Glycine in Brain Tumors at 3T. Magn Res Med. 2016;75:52-62.
6. Choi et al. Measurement of Glycine in the Human Brain in vivo by 1H-MRS at 3T: Application in Brain Tumors. Magn Res Med. 2012;66:609-618.
7. Tiwari et al. Measurement of Glycine in healthy and tumorous brain by triple refocusing MRS at 3T in vivo. NMR Biomed. 2017;30:e3747.
Figures