4916
INVESTIGATION OF CEREBRAL BLOOD FLOW PULSATILITY IN AGING PROCESS USING PHASE CONTRAST MAGNETIC RESONANCE IMAGING1Department of Medical Image Precessing, Amiens University Hospital, BioFlowImage Laboratory/Chimère, University of Picardie Jules Verne, Amiens, France, 2Department of Radiology, Amiens University Hospital, Amiens, France
Synopsis
No study already evaluated how arterial blood flow is transferred from extracranial to intracranial level and how venous outflow is transferred from intracranial sinuses to jugular veins. Healthy young and elderly volunteers were enrolled and underwent phase contrast magnetic resonance imaging to investigate intracranial and extracranial arterial and venous flows. In both groups, we found a significant decrease of arterial and venous flows pulsatilities inside the cranium. However, the intracranial and extracranial cerebral blood flow pulsatilities increased significantly with age.
INTRODUCTION
Two dimensional (2D) phase contrast magnetic resonance imaging (PCMRI) is a non-invasive technique used to quantify fluids velocities and their flow during cardiac cycle. Applied to cerebral system, blood flow parameters in physio-pathological1–5 conditions were assessed and current studies demonstrated that blood flow alterations could lead to many diseases such as vascular diseases, senile dementia2,3. However, little is known about how arterial and venous pulsatilities are transmitted between extracranial and intracranial compartments. We sought the effect of the cranial box and age on blood flow pulsatility in normal conditions along cerebral vascular tree.METHODS
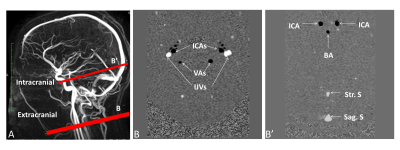
Sixteen healthy young volunteers, HYV (27±4years) and 10 healthy elderly volunteers, HEV (69±5years) underwent PC-MRI to quantify intracranial and extracranial arterial and venous blood flows. PC-MRI data were acquired on 1.5T MRI machine. For the blood flow measurements, a retrospective cardiac-gated phase contrast flow acquisition sequence was used with repetition time: 20ms; echo time: 6ms to 9ms; field of view: 16×16mm2, matrix of 256×128; section thickness: 6mm, flip angle: 25°. Velocity encoding (Venc) was set to 80cm/s and the slices were selected perpendicular to the regions of interest (figure 1). Each acquisition time was approximatively 2 minutes depending on the cardiac period.
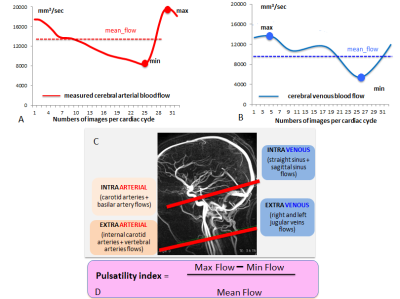
PCMRI data were analysed with Flow software6 to extract the maximal, minimal and mean values of the intracranial arterial blood flow ABF (internal carotid arteries [ICAs] + basilar artery) and venous blood flow VBF (straight + sagittal sinuses) and also extracranial ABF (ICAs + both vertebral arteries) and VBF (both jugular veins). A pulsatility index [PI= (max-min)/mean] was calculated for arterial and venous flows for each intracranial and extracranial level (figure 2).
Shapiro test was used for testing the normality of the different values. Depending on the distribution, we used parametric tests (Student tests) or non-parametric tests (Mann-Whitney or Wilcoxon tests). The results were significant for p< 0.05.
RESULTS
HYV:
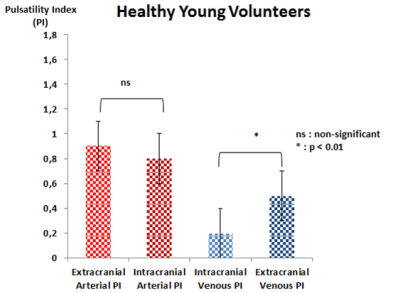
No significant difference (p>0.05) was found between extracranial and intracranial arterial PI. Intracranial venous PI was significantly lower than extracranial venous PI (figure 3).
HEV:
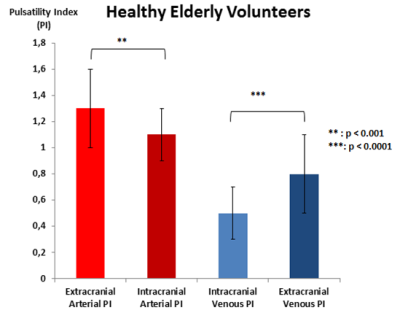
Extracranial arterial PI was significantly (p<0.001) higher than intracranial arterial PI. Intracranial venous PI was significantly (p<0.00001) lower than extracranial venous PI (figure 4).
HYV versus HEV:
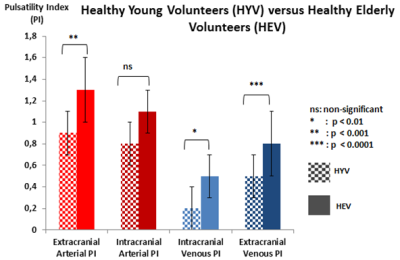
Extracranial arterial PI is significantly (p<0.001) higher in HEV than HYV. Intracranial arterial PI was similar between both groups. Extracranial venous PI and intracranial venous PI were significantly higher (p<0.01) in HEV than HYV (figure 5).
DISCUSSION
2D PCMRI is a quick sequence which can be added to MRI protocol in suspicion of vascular alterations. It provides information about global cerebral blood flow dynamics in fewer 5 minutes. The post-processing of the data is easy, fast, reproducible, and accurate6 if the acquisition was well-done: good cardiac synchronisation, slice perpendicular to the flow direction. With a Venc of 80cm/s, no aliasing usually occurs. However, if needed, antialiasing tool is implemented in the software6.
The intracranial arterial circulation is insufficiently investigated mainly due to the limit of Doppler to assess the blood flow through the cranium7. Close to the Gosling index well known in Doppler investigations8, these new physiological results could be useful to better diagnose cerebral vascular alterations such as arterial hypertension, sinuses thrombosis, vascular dementia…
Previous studies9,10 showed a decrease of flows pulsatilities along of arterial vessels. In this study, we found a decrease of vertebral and internal carotid blood pulsatilities from extracranial to intracranial compartment. Recent works indicate that the brain is vulnerable to increased arterial pulsatility11–13 so that the elasticity of large arteries vessels has a buffering function which decreases pressure and flow pulsatility in the closed cranial box (Windkessel effect). Moreover, the decrease of arterial flow pulsatility could be related to the vessels tortuosity before the Willis polygon, which has a physiologic attenuating effect9,10 on arterial flow pulsatility as already showed.
Studies revealed that an increase of arterial wall stiffness during normal aging affects vascular compliance14. Here, we found that extracranial arterial pulsatility is higher in elderly than younger. In our opinion, young controls presented lower arterial stiffness, higher arterial compliance and less vessels tortuosity between the two levels than elderly population. The probably increase of cerebral resistance1 in elderly makes them less able to dampen aortic flow pulsatility as previously demonstraded15.
Paradoxically, in both groups, as found before16, venous pulsatility increased from sinuses to jugular system suggesting that jugular venous pulsations come from cardiac aspiration than intracranial pressure (ICP).
CONCLUSION
2D PCMRI enables simple, quick and accurate arterial and venous blood flow circulation assessment. Our results showed that the cranial box impacts significantly cerebral blood flow pulsatilities. This impact increases with age.Acknowledgements
No acknowledgement found.References
1. Stoquart-ElSankari, S. et al. Aging effects on cerebral blood and cerebrospinal fluid flows. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 27, 1563–1572 (2007).
2. Bateman, G. A., Levi, C. R., Schofield, P., Wang, Y. & Lovett, E. C. The venous manifestations of pulse wave encephalopathy: windkessel dysfunction in normal aging and senile dementia. Neuroradiology 50, 491–497 (2008).
3. Bateman, G. A. Pulse-wave encephalopathy: a comparative study of the hydrodynamics of leukoaraiosis and normal-pressure hydrocephalus. Neuroradiology 44, 740–748 (2002).
4. Qvarlander, S. et al. Cerebrospinal fluid and blood flow patterns in idiopathic normal pressure hydrocephalus. Acta Neurol. Scand. 135, 576–584 (2017).
5. Baledent, O. et al. Relationship Between Cerebrospinal Fluid and Blood Dynamics in Healthy Volunteers and Patients with Communicating Hydrocephalus. Invest. Radiol. 39, 45–55 (2004).
6. Balédent, O., Henry-Feugeas, M. C. & Idy-Peretti, I. Cerebrospinal fluid dynamics and relation with blood flow: a magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Invest. Radiol. 36, 368–377 (2001).
7. Niederer, P. F. Ultrasound imaging and Doppler flow velocity measurement. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 18, 245–265 (2010).
8. Gosling, R. G. & King, D. H. Arterial assessment by Doppler-shift ultrasound. Proc. R. Soc. Med. 67, 447–449 (1974).
9. Schubert, T. et al. Dampening of Blood-Flow Pulsatility along the Carotid Siphon: Does Form Follow Function? Am. J. Neuroradiol. 32, 1107–1112 (2011).
10. Schubert, T. et al. Attenuation of blood flow pulsatility along the Atlas slope: a physiologic property of the distal vertebral artery? AJNR Am. J. Neuroradiol. 36, 562–567 (2015).
11. Webb, A. J. S. et al. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke 43, 2631–2636 (2012).
12. Wohlfahrt, P. et al. Large artery stiffness and carotid flow pulsatility in stroke survivors. J. Hypertens. 32, 1097–1103; discussion 1103 (2014).
13. Aribisala, B. S. et al. Blood pressure, internal carotid artery flow parameters, and age-related white matter hyperintensities. Hypertens. Dallas Tex 1979 63, 1011–1018 (2014).
14. Safar, M. E. Arterial stiffness: a simplified overview in vascular medicine. Adv. Cardiol. 44, 1–18 (2007).
15. Kwater, A., Gasowski, J., Gryglewska, B., Wizner, B. & Grodzicki, T. Is blood flow in the middle cerebral artery determined by systemic arterial stiffness? Blood Press. 18, 130–134 (2009).
16. Stoquart-Elsankari, S. et al. A phase-contrast MRI study of physiologic cerebral venous flow. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 29, 1208–1215 (2009).
Figures