4909
MRI-based retrospective gating for PET reconstruction in rats: implementation and proof of principle study1Biomedical MRI, KU Leuven, Leuven, Belgium, 2Preclinical Imaging PCI, Bruker Biospin MRI, GmbH, Ettlingen, Germany, 3Preclinical Imaging NMI, Bruker Biospin MRI, GmbH, Valencia, Spain, 4Institute for Instrumentation in Molecular Imaging, i3M-CSIC, Valencia, Spain, 5Nuclear Medicine and Molecular Imaging, KU Leuven, Leuven, Belgium
Synopsis
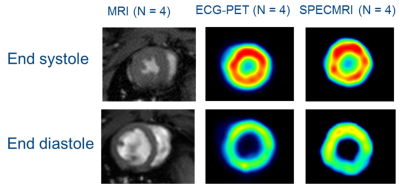
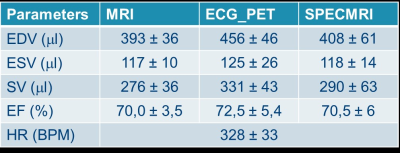
We hereby report the implementation and the preliminary results of an MRI-based retrospective gating strategy for reconstructing simultaneously acquired PET data in rats (SPECMRI). Ejection fraction extracted from MRI, SPECMRI and ECG-gated PET were all within the same range (70.0 ± 3.5%, 72.5 ± 5.4% and 70.5 ±6% for MRI, ECG-gated and SPECMRI respectively). This new technique enables to ease the animal handling (no need for ECG electrodes) and provide true synchronization of PET and MRI data.
Background
While cardiac MRI remains the gold standard for the assessment of left ventricular global function, it lacks sensitivity for molecular imaging strategies. The recent development of preclinical PET insert enables now to bridge the gap and assess simultaneously both global function, tissular integrity and molecular pathways. However the acquisition of gated acquisitions using ECG is subject to many hurdles (corruption of ECG signal by gradient switching, complex animal handling…) that could easily be overcomes using navigator based techniques. The aim of this work was thus to propose a method based on the MRI self-gating technique (IntraGate) (1) to provide the gating information for retrospective PET gated image reconstruction. The benefits of such an approach is a true synchronization of the PET and MRI cardiac acquisition, the easier animal handling (no ECG electrodes required), access to the full cardiac cycle and the possibility to retrospectively assess the quality of the gating information (2). The self-gating imaging technique is also applicable with heavily diseased animals while conventional ECG- triggering fails (3,4).Methods
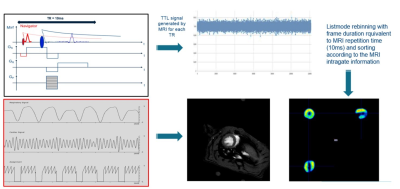
All data were acquired in a BioSpec 70/30 MRI system equipped with a SiPM-based PET insert (Bruker BioSpin) exhibiting about 150 mm axial length. Seven male Wistar rats were used for this study. Animals were not fasted to ensure a good FDG uptake. Anesthesia was induced and maintained through inhalation of 2% Isoflurane carried by 100% oxygen. 18F-FDG (46.1 ± 9.7 MBq) was injected intravenously through the tail vein and the animal placed in a multi-modal MRI compatible animal bed (Bruker Biospin). For MRI, we used a self-gated gradient echo sequence (igFLASH) with the following parameters: TE: 3.585 ms, TR: 10 ms, flip angle: 15 degrees, matrix: 128 x 128 zero filled to 192 x 192, FOV: 60 x 60 mm, 10 contiguous slices of 1.5 mm thickness, oversampling: 250, scan time 53 min 20 s. The MRI sequence was modified to send a TTL signal of 4 ms duration at each TR loop to the PET DAQ electronics. Simultaneously, PET data were acquired using a 1 hr static scan. Then the IntraGate navigator information was used to derive the required retrospective data ordering scheme that represents the position within the cardiac cycle (Figure 1). The list-mode PET data were then rebinned according to the MRI based cardiac cycles to reconstruct 4-16 cardiac PET imaging frames. At the end of the PET-MRI acquisition, an ECG gated PET scan of 20 min was acquired. All PET data were reconstructed using MLEM with 12 iterations without smoothing or PSF modeling included.Results
Out of the 7 datasets acquired, 2 animals had unstable cardiac and respiration rate rendering the processing of the data impossible and another animal had too low activity for reconstructing the PET data. The detection of the TTL signal from the MRI to the PET electronics enabled us to determined precisely the start of the MRI acquisition and each TR loops with an average period of 10.00027 ms (intragate TR being set to 10ms). From the 4 usable dataset, both ECG-gated and MRI-based retrospective gating (SPECMRI) were in agreement with MRI for the extraction of ejection fraction (Table1 and Figure 2) (70.0 ± 3.5%, 72.5 ± 5.4% and 70.5 ±6% for MRI, ECG-gated and SPECMRI respectively).Conclusion
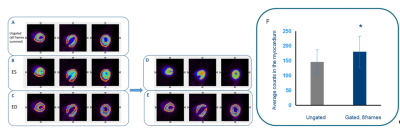
We hereby propose a novel PET/MR method enabling PET cardiac imaging solely based on motion information derived from MRI with high temporal resolution for rodent imaging. Our preliminary results demonstrate the advantage of such technique for true synchronization of PET and MRI data and the potential of using MRI for estimation of ejection fraction error in PET data as well as contribution of partial volume that could explain the differences in uptake values between end diastole and end systole thus correcting for the true quantification of myocardial glucose uptake (Figure 3).Acknowledgements
This research was supported Stichting Tegen Kanker and Hercules foundation.References
1. A. Nauerth, E. Heijman, C. Diekmann. Slice refocusing signal for retrospective reconstruction of CINE cardiac MR images. Proc. Intl. Soc. Mag. Reson. Med. 14 (2006).
2. C. J. Van Echteld, M. G. Nederhoff, B. C. Te Boekhorst, C. W. Van de Kolk, M. A. Jansen, A. Nauerth. Self-gated, wireless cine-MRI allows accurate and time-efficient analysis of mouse heart function when conventional ECG-and respiratory gated cine MRI fails. Proc. Intl. Soc. Mag. Reson. Med. 14 (2006).
3. B. Hiba, N. Richard, H. Thibault, M. Janier. Cardiac and respiratory self-gated cine MRI in the mouse: comparison between radial and rectilinear techniques at 7 T. Magn. Reson. Med., 58 (2007), pp. 745–753.
4. Bovens SM, te Boekhorst BC, den Ouden K, van de Kolk KW, Nauerth A, Nederhoff MG, Pasterkamp G, ten Hove M, van Echteld CJ. Evaluation of infarcted murine heart function: comparison of prospectively triggered with self-gated MRI. NMR Biomed. 2011 Apr;24(3):307-15.
Figures