4907
Initial Validation of Free-Breathing Navigator Gated Cardiac Quantitative Susceptibility Mapping via Comparison with Right Heart Catheterization Measurements1Meinig School of Biomedical Engineering, Cornell University, New York, NY, United States, 2Radiology, Weill Cornell Medicine, New York, NY, United States, 3Medicine, NewYork-Presbyterian, New York, NY, United States, 4Medicine, Weill Cornell Medicine, New York, NY, United States
Synopsis
Our previous work has shown the feasibility of measuring differential RV-to-LV oxygen saturation with cardiac quantitative susceptibility mapping (QSM) in healthy volunteers; in this work, we present our initial validation of cardiac QSM in patients by comparing the QSM-based measurements with gold standard right heart catheter measurements.
Introduction
Quantitative susceptibility mapping (QSM) is an emerging technique for measuring differential RV-to-LV oxygen saturation, which is an established indicator of cardiac performance. Pilot studies have demonstrated feasibility of cardiac QSM in healthy volunteers, but this technique has never before been tested in clinical setting. In this work, we report our experience with post-contrast cardiac QSM and initial validation of oxygenation measurement in patients. Additionally, we present a method to improve cardiac QSM quality by restricting the susceptibility variations in blood pools of the heart.Methods
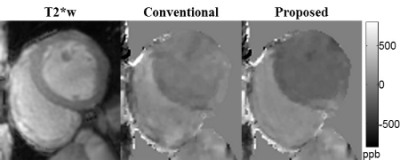
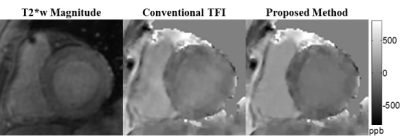
Cardiac QSM was tested in 5 healthy volunteers and 18 patients on a GE 3T scanner using a free-breathing ECG-triggered multi-echo 3D GRE sequence (typical resolution=1.5x1.5x5 mm3, R=2 parallel imaging acceleration). Images were acquired 20-30 min post gadolinium administration in patients (0.2 mmol/kg). QSM maps were reconstructed by first preparing the total field via graph cut phase analysis1,2 and chemical shift update methods3, and then preforming field to source inversion with the total field inversion (TFI) methods4. To improve the quality of cardiac QSM, two additional regularizations were added to TFI to restrict the susceptibility variations within the right (RV) and left ventricle (LV) in similar fashion as MEDI+05:
$$y^*=argmin_{y}\{\frac{1}{2}||w(f-d{\otimes}Py)||^2_2+\lambda||M_G{\triangledown}Py||_1+{\lambda}_{RV}||M_{RV}(Py-\overline{Py_{RV}})||^2_2+{\lambda}_{LV}||M_{LV}(Py-\overline{Py_{LV}})||^2_2\}$$
The first two terms are respectively the data fidelity term and structure consistency regularization terms for TFI4. The last two terms restrict the susceptibility variation in blood, where $$${\lambda}_{RV}$$$ and $$${\lambda}_{LV}$$$ are regularization parameters, $$$M_{RV}$$$ and $$$M_{LV}$$$ are the mask for RV and LV obtained through manual segmentation, and $$$\overline{Py_{RV}}$$$ and $$$\overline{Py_{LV}}$$$ are the average susceptibility in RV and LV, respectively. The QSM map, $$$\chi$$$, is then $$$\chi=Py^*$$$. The QSM maps reconstructed with blood pools regularization were compared with the QSM maps reconstructed without the added regularization in both volunteer and patient data. The differential RV-to-LV oxygen saturation ($$${\Delta}SO_2$$$) was derived from RV-to-LV susceptiblity difference in QSM ($$${\Delta}{\chi}$$$) as described in our previous work6. In 2 patients this was compared with a gold standard right heart catheter measurement (RHC), which was calculated as the oxygenation difference between femoral artery and pumolary artery. QSM maps were also compared with ancillary CMR findings (DE-CMR late-gadolinium enhancement).
Results
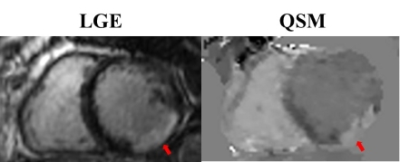
Cardiac QSM was obtained successfully in all healthy subjects and 83% (15/18) of patients. Compared to the conventional TFI, the proposed method with blood regularization improved QSM quality (Figures 1&2) and yielded over 10% higher differential blood susceptibility between RV and LV. In healthy volunteers, $$${\Delta}{\chi}$$$ increased from 230±24ppb to 267±12ppb with the proposed method, which corresponds to an increase in $$${\Delta}SO_2$$$ from 15.8±1.7% to 18.6±0.8%, resulting in better agreement with physiologically norms. In patients, $$${\Delta}{\chi}$$$ was 250±72ppb with conventional TFI, and 309±48ppb with proposed method. In the 2 patients who underwent RHC (within 3 weeks of CMR), $$${\Delta}SO_2$$$ obtained by the proposed QSM agreed reasonably well with RHC measurements in patient 1 (30.8% vs. 29%), but underestimated in patient 2 (28.5% vs. 35%). In the remaining 13 patients, $$${\Delta}SO_2$$$=21.7±2.3%, which was within the physiologically normal range. In patients imaged post-contrast, myocardium was found to have lower susceptibility than LV blood, which is consistent with the expected higher gadolinium concentration in LV blood compared with myocardium7 (figure 2). Ancillary analyses also demonstrated altered myocardial tissue properties on QSM, including increased susceptibility corresponding to transmural late gadolinium enhancement on DE-CMR (Figure 3).Disscussion
The current approach to cardiac QSM observed an 83% success rate in patient, which can be further improve by reducing scan time (typical scan time=500±198 s) through higher order parallel imaging acceleration. The values in the QSM maps were also underestimated, suggesting that the regularization parameters and preconditioner in the QSM reconstruction algorithm may need to be better optimized to account for the large susceptibility difference between the heart and its neighboring air in the lung.Conclusion
Our preliminary data suggest that free-breathing cardiac QSM is feasible in patients and provides comparable blood oxygenation measurements without requiring invasive catheterization. Further research is needed to improve the accuracy and success rate of free-breathing cardiac QSM.Acknowledgements
This work was supported in part from NIH grant R01HL128278, R01NS072370, R01NS090464, and R01NS095562.References
1. Dong, J., et al., Simultaneous Phase Unwrapping and Removal of Chemical Shift (SPURS) Using Graph Cuts: Application in Quantitative Susceptibility Mapping. IEEE Transactions on Medical Imaging, 2015. 34(2): p. 531-540.
2. Reeder, S.B., et al., Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magnetic Resonance in Medicine, 2004. 51(1): p. 35-45.
3. Dimov, A.V., et al., Joint estimation of chemical shift and quantitative susceptibility mapping (chemical QSM). Magnetic Resonance in Medicine, 2015. 73(6): p. 2100-2110.
4. Liu, Z., et al., Preconditioned total field inversion (TFI) method for quantitative susceptibility mapping. Magnetic Resonance in Medicine, 2016: doi:10.1002/mrm.26331.
5. Liu, Z., et al., MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping. Magnetic Resonance in Medicine. doi:10.1002/mrm.26946
6. Wen, Y., et al., Cardiac quantitative susceptibility mapping (QSM) for heart chamber oxygenation. Magnetic Resonance in Medicine. doi:10.1002/mrm.26808
7. Goldfarb, J.W., S. Arnold, and M. Roth, Gadolinium pharmacokinetics of chronic myocardial infarcts: Implications for late gadolinium-enhanced infarct imaging. Journal of Magnetic Resonance Imaging, 2009. 30(4): p. 763-770.
Figures