4905
Cardiac and Respiratory Self-Gated Motion-Corrected Free-Breathing Spiral Cine Imaging.1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Medicine, Cardiovascular Division, University of Virginia, Charlottesville, VA, United States, 3Medicine, Cardiovascular Medicine, University of Virginia, Charlottesville, VA, United States, 4Medicine, Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States
Synopsis
We developed a free-breathing continuous-acquisition respiratory and cardiac self-gated spiral cine pulse sequence. Data was acquired using a single spiral interleaf rotated by the golden-angle in time. The cardiac self-gating signal was extracted using principal component analysis on a gridded 8x8 central region of k-space for each spiral, and the respiratory motion is derived from rigid registration for each heartbeat. Images were reconstructed with motion compensated SPIRiT using 16 seconds (2000 spirals) or 8 seconds of data. Free-breathing self-gated spiral cine imaging demonstrated high image quality providing whole heart coverage with clinical spatial and temporal resolution in under 3 minutes.
Introduction
In current clinical practice, breath-held ECG-gated Cartesian cine images are typically acquired to assess cardiac function. This approach is inefficient as it requires 10-12 breath-holds to cover the left ventricle, and it is susceptible to both respiratory-motion and ECG gating artifacts, particularly at 3T. Thus, there is a growing interest in self-gated free-breathing approaches. We developed a continuous-acquisition respiratory and cardiac self-gated cine sequence for free-breathing cardiac function acquisition, and developed a motion-compensated reconstruction strategy.Methods
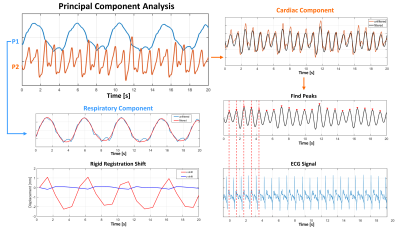
22 volunteers were imaged on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). Gradient echo cine data were acquired continuously for 16 seconds per slice (2000 spirals) using a pulse sequence consisting of a single spiral out trajectory rotated by the golden angle. Sequence parameters included: TR 7.8 ms, TE 1 ms, slice thickness 8 mm, resolution 1.5x1.5 mm2. Self-gating respiratory and cardiac signals were determined by gridding an 8x8 central region of k-space for each spiral arm for all coils, followed by low-pass temporal filtering, principal component analysis, and band-pass filtering of the derived temporal-basis functions (Figure 1). The accuracy of self-gating was compared to the acquired ECG signal with Bland-Altman analysis. For each heartbeat, a static image was reconstructed using all spiral arms following each self-gating trigger. These images were rigidly-registered to derive the respiratory motion.1 The cardiac self-gating signal was used to retrospectively bin the data across the cardiac cycle. We evaluated three spiral designs: (1) uniform density spiral (UD), (2) linear variable density (VD), and (3) dual density spiral with a broad transition region (DD) in 2 subjects. Cine images were reconstructed using data from the whole 16 seconds acquisition (2000 spirals) using non-Cartesian SPIRiT 2 with a reconstructed temporal resolution of 40 ms. To evaluate the performance with a shorter acquisition, image reconstruction was also performed using only half of the data (8 seconds, 1000 spirals). Data from the first 200 spirals were discarded to allow the signal to reach steady state. Images were graded on a 5-point scale (1-worst, 5-best) by an experienced cardiologist. Cine images covering the whole heart were acquired in 8 subjects. Ejection fraction was calculated by a cardiologist from the images acquired using a standard ECG gated SSFP breath-hold cine sequence and our proposed strategy with both 16 or 8 seconds of data.Results
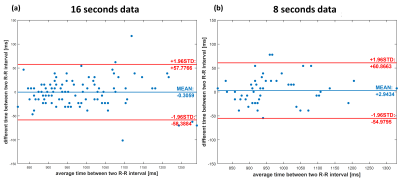
Figure 1 shows the cardiac and respiratory self-gating pipeline. The derived respiratory component was consistent with the respiratory signal derived from rigid registration, and the cardiac cycle lengths matched those of the ECG. Figure 2 shows the 3 spiral trajectories that were evaluated and example images from one subject at end systole and end diastole. The dual-density spiral (DD) reconstruction demonstrated the least amount of residual aliasing and was used in the remaining cases. Figure 3 showed the Bland-Altman plot of the RR intervals as determined by the self-gating strategy as compared to the ECG signal. The self-gating performed similarly for both 16 seconds and 8 seconds of data with a low STD of 25ms, (which was 5% of the average RR interval. Figure 4 shows the image results from one subject at different phases of the cardiac cycle for 16 seconds and 8 seconds worth of data. As expected, there was more residual aliasing with 8 seconds of data. Figures 5 show images at end diastole and end systole covering the whole heart in one subject with 16 seconds and 8 seconds of data. The mean EF’s determined from the 3 techniques were 59.66%, 58.38%, and 57.08%. The mean image quality scores for the 16 seconds and 8 seconds of data were 3.9±0.7 and 3.6±0.5 respectively (P<0.05).Conclusion
Our self-gated free-breathing spiral cardiac cine imaging strategy acquired high quality cine images with temporal and spatial resolution typical for breath-held cine imaging without the need for ECG gating or breath-holding. This strategy could provide a simpler, more efficient protocol for clinical CMR imaging particularly for patients who may have difficulty holding their breath.Acknowledgements
This work was supported by NIH K23 HL112910 and R01 HL079110.References
1. Huang W, Yang Y, Chen X, et al. Simple motion correction strategy reduces respiratory-induced motion artifacts for k-t accelerated CMR perfusion imaging [abstract]. Proceedings of the 23rd ISMRM Annual Meeting and Exhibition; 2015 May 30 - June 5; Toronto, Ontario, Canada.
2. Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn. Reson. Med. 2010;64:457–471. doi: 10.1002/mrm.22428
Figures