4904
Simultaneous T1 and T2 Mapping of the Carotid Artery with T2 and Inversion Recovery Prepared 3D Radial Imaging1Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Department of Radiology, University of Washington, Seattle, WA, United States, 3Philips Research China, Shanghai, China
Synopsis
Multi-contrast MRI has been widely used for comprehensive characterization of atherosclerotic plaque. However, the qualitative nature and long scan time have limited its clinical application. In this study, a 3D high spatial resolution time-efficient technique, iGOAL-SNAP, is proposed, which generates different T1 and T2 contrasts in a single scan of 8min, enabling simultaneous T1 and T2 mapping. In addition, a rapid B1 correction method is presented in the fitting process. The accuracy and feasibility of iGOAL-SNAP was demonstrated using phantoms and in vivo studies.
Introduction
Previous work has established the capability of multi-contrast vessel wall MRI in differentiating plaque components in carotid arteries (1). However, due to the qualitative nature of multi-contrast imaging, plaque components are identified by comparing to the signal intensity of reference tissues, which is subjective resulting in intra/inter-reader variability and strongly influenced by imaging parameters and coil positioning (2). Quantitative mapping of carotid vessel wall has been reported (3-5). However, these techniques will require separate scans to obtain T1 and T2 maps, which increases total scan time and is prone to misregistration errors. In this study, we aimed to develop a technique that allows simultaneous T1 and T2 mapping of carotid vessel wall with large coverage and 3D isotropic resolution.Methods
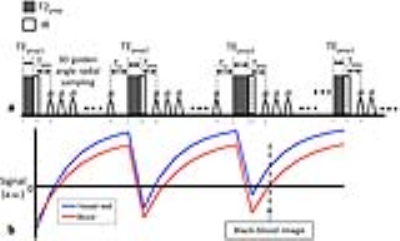
Sequence Design: A previously described sequence for fast 3D T1 mapping (GOAL-SNAP) (5) was improved in this study to allow simultaneous T1 and T2 mapping and named iGOAL-SNAP. As shown in Fig. 1a, iGOAL-SNAP is prepared with both adiabatic T2 preparation (6) with variable duration (TEprep), and IR pulses (T2IR) to produce T1 and T2 contrast. Following T2IR preparation, 3D radial sampling is adopted for isotropic acquisition, and water excitation pulse is used for fat suppression. The 3D golden angle radial order of the GOAL-SNAP sequence (5) was modified for iGOAL-SNAP to achieve uniform distribution of spokes used for reconstruction at a certain TI and TEprep.
Image reconstruction and T1, T2 fitting: Sliding window reconstruction was performed to reconstruct the T2IR image series by combining spokes of all shots with the same TI and TEprep. CG-SENSE iteration (7) was used to reduce undersampling artifacts with the sensitivity maps estimated by using spokes at TI larger than 1300ms. To take into account B1 inhomogeneity, another two 3D radial SPGR images were acquired with flip angles different from iGOAL-SNAP (8). Then, five parameters including proton density, inversion efficiency, B1, T1 and T2 were estimated simultaneously by fitting the signals of the T2IR image series and the SPGR images to the signals simulated using Bloch equation. The imaging parameters of iGOAL-SNAP and 3D radial SPGR are shown in Table 1. In this study, the temporal width for the sliding window reconstruction of the T2IR image series was set 25 (250s/frame), resulting in 21 frames for T1/T2 fitting. Additionally, a black-blood image with near-zero luminal signal (Fig. 1b) was reconstructed to depict vessel wall (iGOAL-SNAP VW), using the KWIC method (9).
MR Imaging: All scans were performed on a 3T MR scanner (Philips, Achieva). First, iGOAL-SNAP was tested in phantoms with different T1 and T2 values. The standard 2D T1 and T2 mapping sequences: IR-spin echo (IR-SE) and multi-echo spin echo (ME-SE), were performed for comparison. After institutional review board approval, in vivo imaging was performed in five heathy volunteers and five patients with carotid atherosclerosis. In healthy volunteers, iGOAL-SNAP was compared with 2D Molli (10) and T2prep-bSSFP (11) in measuring T1 and T2 of cervical muscles. In patients with carotid atherosclerosis, iGOAL-SNAP was compared with conventional multi-contrast imaging protocol (1) and SNAP (12).
Results
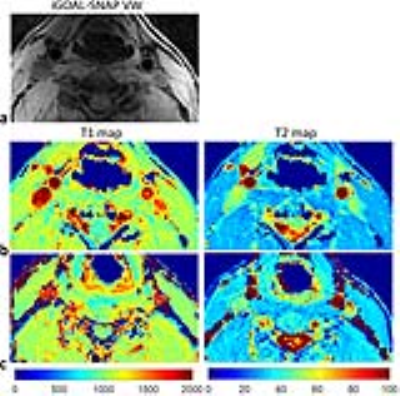
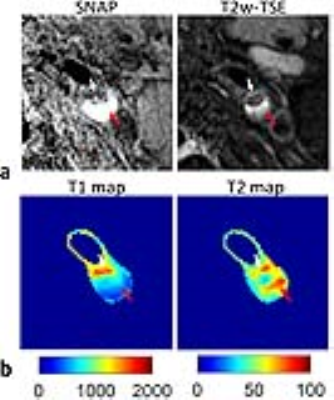
The mean T1 and T2 values of phantoms from iGOAL-SNAP were in good agreement with 2D IR-SE and ME-SE (T1: R2=0.99; T2: R2=0.99, Fig. 2a). Bland-Altman plots showed the measurement bias with the average of the measurements (Fig. 2b). Figure 3 shows the example images of a healthy volunteer, where vessel wall can be clearly seen on the iGOAL-SNAP VW. The T1 and T2 of muscle in the five volunteers estimated by iGOAL-SNAP (T1: 1027±32.3ms; T2: 30.3±1.1ms) were reasonable compared with Molli (1012±22.1ms) and T2prep-bSSFP (31.8±2.1ms). The mean T1 and T2 values of carotid vessel wall from the ten arteries in healthy volunteers were 1213±48.3ms and 51.1±1.7ms, respectively. Eight carotid plaques were found in the five patients. IPH was detected in five plaques on SNAP images. Figure 4 shows an example of carotid plaque with IPH. Alternations of T1 and T2 values observed in the plaque region were in agreement with the findings of SNAP and T2-weighted images.Discussion and Conclusions
A high spatial resolution, time-efficient, simultaneous vessel wall T1 and T2 mapping technique was developed, which showed excellent correlation in measuring T1 and T2 of phantoms with traditional 2D SE methods. Preliminary in vivo studies indicated good feasibility and great potentials of the proposed technique for comprehensive characterization of carotid plaque in a single scan. Compared with existing T1-weighted and T2-weighted techniques, iGOAL-SNAP may provide more quantitative, reproducible biomarkers of carotid atherosclerosis.Acknowledgements
Supported by the National Natural Science Foundation of China (81371540, 81571667) and the National Institutes of Health (R01 HL103609).References
1. Cai JM, Hastukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106(11):1368-1373.
2. Li FY, Yarnykh VL, Hatsukami TS, Chu BC, Balu N, Wang JN, Underhill HR, Zhao XH, Smith R, Yuan C. Scan-Rescan Reproducibility of Carotid Atherosclerotic Plaque Morphology and Tissue Composition Measurements Using Multicontrast MRI at 3T. J Magn Reson Imaging 2010;31(1):168-176.
3. Biasiolli L, Lindsay AC, Chai JT, Choudhury RP, Robson MD. In-vivo quantitative T2 mapping of carotid arteries in atherosclerotic patients: segmentation and T2 measurement of plaque components. J Cardiovasc Magn Reson 2013;15:69.
4. Coolen BF, Poot DH, Liem MI, Smits LP, Gao S, Kotek G, Klein S, Nederveen AJ. Three-dimensional quantitative T1 and T2 mapping of the carotid artery: Sequence design and in vivo feasibility. Magnetic resonance in medicine 2016;75(3):1008-1017.
5. Qi H, Sun J, Qiao H, Chen S, Zhou Z, Pan X, Wang Y, Zhao X, Li R, Yuan C, Chen H. Carotid Intraplaque Hemorrhage Imaging with Quantitative Vessel Wall T1 Mapping: Technical Development and Initial Experience. Radiology 2017;(Accepted).
6. Nezafat R, Stuber M, Ouwerkerk R, Gharib AM, Desai MY, Pettigrew RI. B1-insensitive T2 preparation for improved coronary magnetic resonance angiography at 3 T. Magn Reson Med 2006;55(4):858-864.
7. Pruessmann KP, Weiger M, Bornert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med 2001;46(4):638-651.
8. Kecskemeti S, Samsonov A, Hurley SA, Dean DC, Field A, Alexander AL. MPnRAGE: A technique to simultaneously acquire hundreds of differently contrasted MPRAGE images with applications to quantitative T1 mapping. Magn Reson Med 2016;75(3):1040-1053.
9. Song HK, Dougherty L. k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med 2000;44(6):825-832.
10. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52(1):141-146.
11. Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 2009;11:56.
12. Wang JN, Bornert P, Zhao HL, Hippe DS, Zhao XH, Balu N, Ferguson MS, Hatsukami TS, Xu JR, Yuan C, Kerwin WS. Simultaneous noncontrast angiography and intraPlaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med 2013;69(2):337-345.
Figures