4903
Propagation of Metaplastic Adipose Tissue Throughout the Scar of Hemorrhagic Myocardial Infarct is an Iron-Dependent Process: Cardiac MRI Study with Histological Insights1Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2University of Wisconsin, Madison, WI, United States
Synopsis
Lipomatous metaplasia (LM) of myocardial infarctions (MI) is typically observed in the peripheral zone of chronic MI and has been linked to major adverse clinical outcomes. To date, the mechanisms driving LM of MI remain unknown. A common feature of many disease processes associated with pathological fat accumulation is the iron-induced foam cell formation. Growing body of evidence now shows that iron deposits within hemorrhagic MI drive the prolonged recruitment of phagocytes into the infarcted territory. Herein, we investigated the spatial distribution and temporal accumulation of fatty infiltration in hemorrhagic MIs.
INTRODUCTION
Based on autopsy reports and imaging studies over the last 30 years, it is estimated that fatty infiltration of post-MI scar shows an incidence of >50%. Fat depots are typically observed in the peripheral and border zones of old scars and have been linked to major adverse clinical outcomes in the late phase of chronic MI. The mechanism behind this phenomenon has conventionally been attributed to the process of “lipomatous metaplasia”.
Atherosclerosis studies have taught us that upon phagocytosis of oxidized red blood cells (oxRBC), iron-laden macrophages (siderophages) tend to oxidize surrounding LDL, accumulate cholesterol, and transform into foam cells. The process of foam cell formation following erythrophagocytosis is accompanied by exocytosis of iron. Phagocytosis of this exocytosed iron by new macrophages can lead to a self-perpetuating and amplifying loop of foam cell formation. In this study, we investigated the spatial distribution and temporal accumulation of fatty infiltration in hemorrhagic MIs.
METHODS
Hemorrhagic MI was created in 20 dogs by 3-hour occlusion of the LAD artery followed by 8-week (n=10) or 6-months (n=10) reperfusion. All dogs underwent cine, T2* and LGE MRI in a 3T clinical MRI system (MAGNETOM Verio, Erlangen, Siemens Healthcare) on day 7, week 8, and month 6 post-MI. ECG-triggered breath-held 2D cine-SSFP images (20-25 cardiac phases, TR/TE=3.5/1.75 ms, flip angle=70°, BW=930 Hz/pixel), T2*-weighted images (multiple gradient-echo, TR=12ms, 6 TEs=2.0ms–9.5ms with ΔTE=1.5ms, flip angle=10° and BW=930Hz/pixel), and LGE images (IR-FLASH acquired 10-15 minutes following intravenous gadolinium infusion (Magnevist, Bayer Healthcare Pharmaceuticals Inc., Wayne, NJ), optimal TI to null remote myocardium, TR/TE=3.0/1.5 ms, flip angle=25°, BW=586 Hz/pixel) were acquired along the short-axis direction covering the entire LV. Commonly used imaging parameters for all the scans were: resolution=1.4 x 1.4 x 6 mm3. Left ventricular ejection fraction (EF) and end-diastolic sphericity index (EDSI) were determined from cine images at week 8 and month 6 post-MI. All quantitative image analyses were performed using the cvi42 (Circle Cardiovascular Imaging Inc.). Animals were euthanized following week 8 or month 6 MRI scan. Subsequently, the hearts were explanted for histological evaluation of lipomatous metaplasia.RESULTS
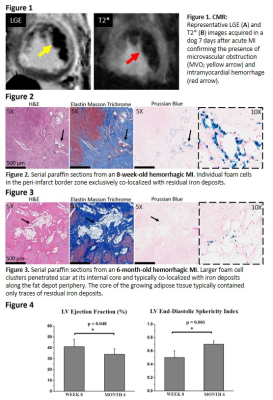
Reperfusion hemorrhage (Figure 1), chronic iron deposits (Figures 2 & 3), and lipomatous metaplasia (Figures 2 & 3) were detected in all dogs. There was no change in iron concentration (1/T2*) within MIs (day 7 to month 6 post-MI (P=0.12)). At week 8 and month 6 post-MI, individual foam cells and mini-clusters of foam cells were typically observed in the peripheral and border zones of hemorrhagic MIs where foam cells exclusively colocalized with iron deposits (Figure 2). At month 6, larger fat depots which penetrated scar tissue at its internal core (Figure 3) were observed. Notably, these large foam-cell clusters typically colocalized with iron deposits along the fat depot periphery, while the core of the growing adipose tissue was found without iron deposits. Propagation of lipomatous metaplasia throughout the scar core was significantly correlated with adverse ventricular remodeling, as evidenced by significant decrease in left ventricular EF and increase in EDSI between week 8 and month 6 (Figure 4).DISCUSSION
Here we demonstrate that fatty degeneration of hemorrhagic MI begins in the peripheral region of the infarcted myocardium and gradually invades the scar core. Moreover, our data suggest that LM of hemorrhagic MI is a self-perpetuating process driven by foam cell formation and iron recycling. The most striking evidence supporting our argument is that large fat depots in 6-month old scars colocalized with iron deposits primarily along the adipose tissue border, while the core of adipose tissue contained only traces of iron.