4901
3D myocardial t1 mapping using a 2D fat image navigator for respiratory motion correction1School of Biomedical Engineering and Imaging Science, King's College London, London, United Kingdom, 2Philips Healthcare, London, United Kingdom
Synopsis
In this study, we propose a 3D whole-heart saturation-recovery T1-mapping technique combined with a 2D fat image navigator (fat-iNAV) for respiratory motion compensation. Respiratory motion of the heart is estimated from the 2D fat-iNAVs and used to correct the T1-weighted images prior to the fitting. The delineation of the myocardium is considerably improved after motion correction, while gating permits to further improve image quality and precision (p<0.05) of the T1 maps. Future work will focus on validating the proposed technique on patients with cardiovascular disease.
Introduction
Myocardial T1 mapping has emerged as a novel and non-invasive method to assess both focal and diffuse myocardial fibrosis. While current clinical implementations are 2D and acquired in a breath-hold, 3D T1 mapping allows for complete coverage of the heart with higher signal to noise ratio and image resolution than feasible with 2D acquisitions. A 3D saturation-recovery T1 mapping technique, called 3D SASHA1,2, has been proposed recently. A 1D diaphragmatic navigator was used to gate and correct for respiratory motion and to enable free breathing acquisition. Scan time however is often unpredictable in patients with irregular breathing. Here we propose to combine the 3D SASHA sequence with a 2D image navigator3 with fat excitation to allow for model independent motion correction with near 100% scan efficiency and higher image resolution.Methods
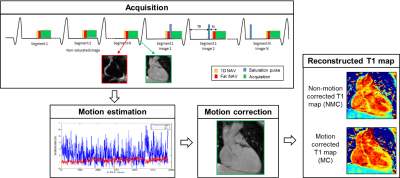
Data from six healthy subjects were acquired on a 1.5T MR scanner (Ingenia, Philips, The Netherland). The acquisition and reconstruction framework is shown in Figure 1. A segmented k-space 3D SASHA acquisition was used with the following acquisition parameters: TR/TE=3.2/1.6, FA=35°, subject specific mid-diastolic trigger delay, image resolution=2x2x4mm3, FOV=300x300x90mm3, SENSE=2. For respiratory motion compensation, a 2D low resolution (3x3x20mm3) image navigator was acquired before each 3D SASHA k-space segment using fat-only excitation (2D fat-iNAV) to render it less sensitive to signal changes due of the varying saturation times of the SASHA sequence. As fat signal recovers faster than the water signal it is consequently not heavily affected by the contrast change between the different T1-weighted images. The 2D fat-iNAV acquisition parameters included: TFEPI acquisition, EPI factor=9, TFE factor=5, image resolution=3x3x20mm3, flip angle=15˚, fat-selective 1331 binomial pulse. 2D foot-head and right-left translational motion was estimated from the 2D fat-iNAV and used to correct each T1-weighted image in a beat-to-beat fashion. To investigate the incremental value of motion correction only versus motion correction with outlier rejection, conventional 1D navigator (1D NAV) acquisitions with a fixed gating window of 10mm and 100mm (ungated) were also performed. Mean and standard deviation were measured in a selected ROI in the myocardium before and after motion correction as an estimate of accuracy and precision.Results
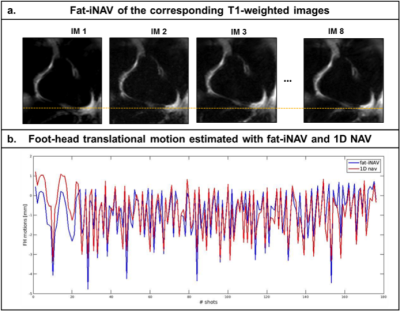
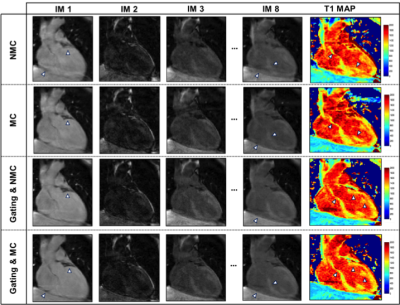
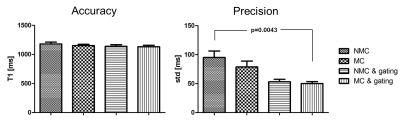
The 2D fat-iNAVs are not affected by the change in contrast for acquisitions with images at different saturation delay times, as shown in Figure 2a. There was a good correlation (r=0.81) of the foot-head motion information estimated with the fat-iNAV and the 1D NAV (respectively in blue and red in Figure 2b). Figure 3 shows the single T1-weighted images and the corresponding reconstructed myocardial T1 maps from one volunteer. Prior to any motion correction (NMC) the T1 map is severely affected by respiratory motion. The delineation of the myocardial borders significantly improves after translational motion correction with the 2D fat-iNAV (MC) and 100% scan efficiency. Image quality is similar if gating only is applied using a 1D NAV (NMC and 10 mm gating) but at the expense of increased scan time. If gating is combined with translational motion correction estimated from the 2D fat-iNAV (MC and 10mm gating) delineation of the myocardium and papillary muscles further improves. The precision significantly improves (p<0.05) with 10mm gating and motion correction compared to the NMC T1 map whereas the accuracy is maintained (p=0.31), as shown in Figure 4.Conclusion
We demonstrate the feasibility of combining 3D SASHA T1 mapping sequence with a 2D fat-iNAV for respiratory motion compensation. The fat-iNAV was not affected by the contrast change of the T1 mapping acquisition and permits to correctly estimate and correct for the respiratory motion of the heart. Future work will focus on investigation further non-rigid motion correction for 100% scan efficiency fat-iNAV and validating the proposed technique on patients with cardiovascular disease.Acknowledgements
This work was supported by the EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1), Philips Healthcare an EPSRC programme and project Grant (EP/P001009/1 and EP/P007619/1) and FONDECYT N° 1161051.References
1 Chow K, et al. Saturation recovery single shot acquisition (SASHA) for myocardial T(1) mapping. Magn Reson Med 2014;71:2082–2095.
2 Nordio G, et al. 3D myocardial T1 mapping using saturation recovery. Journal of Magnetic Resonance Imaging. 2017;46(1):218-227.
3 Henningsson M, et al. Prospective respiratory motion correction for coronary MR angiography using a 2D image navigator. Magn Reson Med 2013; 69:486-494
Figures