4893
3D Radial Free-breathing Variable Flip Angle Whole Heart Myocardial T1 Mapping1Departments of Medical Physics and Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
A number of quantitative T1 mapping techniques have been developed for assessing myocardial pathologies. Inversion recovery (IR) techniques such as MOLLI are widely used due to higher precision and better reproducibility but tend to underestimate T1 values. The aim of this study was to evaluate the potential of new, free-breathing, variable flip angle (VFA), 3D radial and 3D hybrid-radial-Cartesian IR techniques accelerated using radial k-space undersampling for myocardial T1 quantification. Our initial results show promise and suggest that the proposed 3D T1 mapping techniques are not sensitive to B1 variations.
INTRODUCTION
T1 mapping is a promising technique to detect and quantify both focal and diffuse myocardial fibrosis at an early stage. Currently, several techniques are used for assessing myocardial pathologies. Inversion recovery (IR) techniques such as MOLLI1 are widely used due to higher precision and better reproducibility, but tend to underestimate T1 due to heart rate dependence and range of T1 values. In practice, separate MOLLI protocols optimized for different heart rates and pre/post contrast T1 values are utilized. Saturation recovery (SR) methods such as SASHA2 can offer improved accuracy with less precision (i.e. less reproducible) despite being more sensitive to noise. Complete coverage of the whole heart with these typically 2D breathhold methods requires multiple (12-16) short-axis slices, each requiring a 10-20 second breathhold. Even in the most cooperative patients who can hold breaths, imaging the whole heart typically requires 8-10 minutes with longer scan times more common in less cooperative patients. Each current method has its strengths and weaknesses in terms of accuracy, precision, and reproducibility. In this work, we present two new, free-breathing, variable flip angle (VFA) inversion recovery (IR) myocardial T1 mapping techniques (3D radial and 3D hybrid-radial-Cartesian / stack-of-stars (SOS) ) that are accelerated using radial k-space undersampling and robust to variations in B1.METHODS
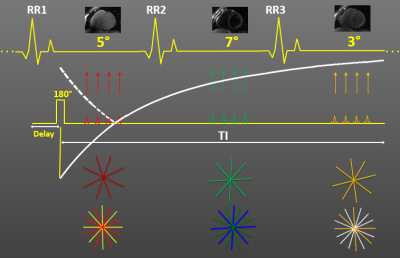
All studies were performed on a 3 T scanner (GE Healthcare, Waukesha, WI). We have implemented free-breathing, ECG-gated, 3D radial and 3D radial-Cartesian (stack-of-stars) spoiled gradient echo (SPGR) IR techniques to investigate potential benefits for myocardial T1 quantification3. Inversion pulses are applied every three heartbeats and imaging is performed with an alternating variable flip angle approach using a flip angle of 5o in the first heartbeat, 7o in the second, and 3o in the third. Figure 1, for simplicity, shows only one image acquisition per heartbeat but depending on scan parameters, typically 57-65 images per heartbeat are acquired for a total of 171-195 images with different inversion times. Respiratory motion is mitigated by employing a modified diminishing variance algorithm (DVA) with 50 % efficiency. 3D radial acquisitions were performed with FOV=25.6x25.6x25.6 cm, 2 mm isotropic resolution, flip angle=5,7,3o, BW=±62.5kHz, TR/TE=3.05/1.08 ms, acquisition window per heartbeat=200 ms, and scan time=9 min and 3D SOS acquisitions with FOV=25.6x25.6x12.8 cm, 2x2x8 mm resolution, flip angle=5,7,3o, BW=±62.5kHz, TR/TE=3.5/1.09 ms, acquisition window per heartbeat=200 ms, and scan time=6 min. After validation of the proposed techniques against a fast spin echo (FSE) IR technique in phantom studies, human studies were performed. Pixel-wise T1 maps obtained using a theoretical signal model were compared to those obtained with MOLLI. Precision was determined from the standard deviation in a region-of-interest (ROI) over myocardium. The effect of flip angle choice and order on T1 measurements were also investigated.RESULTS AND DISCUSSION
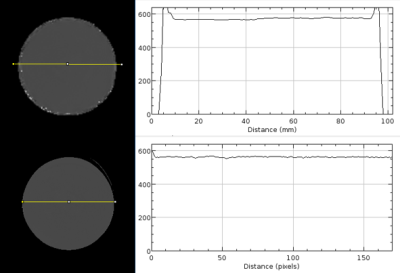
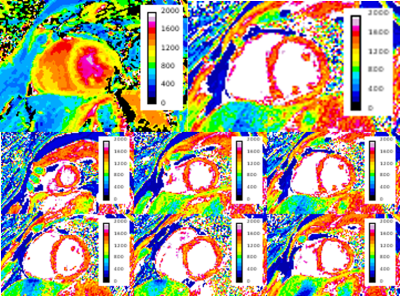
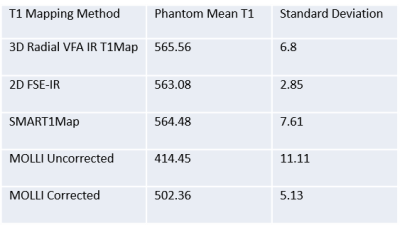
Table 1 shows the accuracy and precision of various T1 mapping methods in a uniform phantom. T1 values obtained with the 3D radial method show good agreement with FSE-IR and SMART1Map4 values and differ with MOLLI T1 values, which are significantly lower. Note that the phantom T1 value chosen (~565 ms) mimics T1 values expected at 3 T after Gd administration5. As seen in Figure 2, T1 profile obtained with our technique across the phantom is very uniform showing B1 inhomogeneity compensation and is in good agreement with that of FSE-IR. Future of MRI belongs to quantitative methods that are not only robust but also accurate for the delineation of disease extent. Figure 3 shows short-axis myocardial native T1 maps from a healthy volunteer. Mean T1 values for MOLLI and our 3D method are 1137±104 ms, and 1447±148 ms, respectively. While it's known that MOLLI underestimates T1 values, it is still widely used due to its reproducibility and robustness. Our native T1 values in healthy subjects are closer to previously reported values at 3 T6. Our current reconstruction method has not yet utilized parallel imaging or exploited spatio-temporal information using compressed sensing, but these approaches are likely to further reduce the variance of T1 maps and therefore improve quantification. Flip angle choice (3,5,7 or 3,6,9) or order (3,5,7 or 5,7,3 or 7,5,3) studied in phantom s didn’t have any significant effect on T1. Additionally, our free-breathing 3D radial method should simplify scan prescription and reduce patient discomfort while providing whole heart coverage.CONCLUSION
Although its applicability has yet to be validated in patient studies, the proposed free-breathing, ECG-gated, 3D radial VFA IR T1 mapping techniques yielded results similar to native myocardial T1 values cited in literature. Our initial results show promise for native myocardial T1 and extracellular volume fraction (ECV) quantification. Further studies are underway to optimize and investigate potential benefits in patients.Acknowledgements
No acknowledgement found.References
1. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, and Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high resolution T1 mapping of the heart. MRM 2004;52:141.
2. Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, and Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T1 mapping. MRM 2013;71:2082.
3. Kecskemeti S, Johnson K, Francois C, Schiebler M, and Unal O. Volumetric Late Gadolinium-Enhanced Myocardial Imaging with Retrospective Inversion Time Selection. JMRI 2013;38:1276.
4. Slavin GS, Stainsby JA. True T1 mapping with SMART1Map (saturation method using adaptive recovery times for cardiac T1 mapping): a comparison with MOLLI. JCMR 2013;15:3.
5. Sharma P, Socolow J, Patel S, Pettigrew RI, and Oshinski JN. Effect of Gd-DTPA-BMA on blood and myocardial T1 at 1.5T and 3T in humans. JMRI 2006;23:323.
6. Weingärtner S, Meßner N, Budjan J, Loßnitzer D, Mattler U, Papavassiliu T, Zöllner F, and Schad L. Myocardial T1-mapping at 3T using saturation-recovery: reference values, precision and comparison with MOLLI. JCMR 2016;1:84.
Figures