4890
Contrast-free 3D whole-heart magnetization transfer imaging for simultaneous myocardial scar and cardiac vein visualization1School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
A novel 3D contrast-free and motion corrected sequence for simultaneous assessment of chronic myocardial scar and coronary veins is proposed, using magnetization transfer ratio (MTR) to target the increase in collagen content associated with fibrosis. Two gradient echo datasets are sequentially acquired to obtain MTR: a reference and an off-resonance MT-weighted image. Bloch simulations and in-vivo data demonstrated that the proposed acquisition is superior to bSSFP MT-weighted sequences, yielding more consistent MTR values throughout the myocardium. Scans in patients with chronic scar confirmed the ability of MTR to localize scar, in addition to cardiac vein visualization from the MT-weighted image.
Purpose
Identification of fibrotic tissue in myocardium is important for the management of cardiac arrhythmia, patient risk stratification and planning of ablation procedures, among others. Late Gadolinium Enhancement (LGE) MRI is the gold standard for assessment of fibrosis but requires the injection of a contrast agent. As intravenously administered gadolinium accumulates in neural tissue [1] and is associated with increased risk of nephrogenic systemic fibrosis in patients with severe renal dysfunction, a non-contrast approach would be desirable. Magnetization transfer (MT) is an endogenous MR contrast mechanism, shown to be sensitive to structural changes associated with myocardial fibrosis, such as the increase in collagen content in myocardium following an infarct [2-4]. Furthermore, MT pre-pulses could be exploited for cardiac vein visualization [5] in the same examination, which may be beneficial in patients requiring cardiac resynchronization therapy, where characterization of coronary sinus/vein anatomy and ventricular fibrosis/viability can help to guide intervention. In this work, we exploit MT for simultaneous assessment of localized fibrosis and coronary vein anatomy.Methods
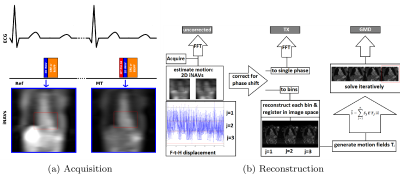
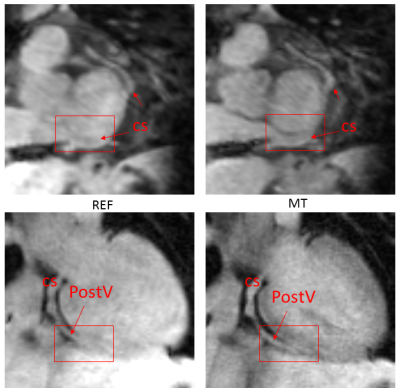
A prototype 3D motion corrected free-breathing imaging sequence was devised and implemented on a 1.5T MR scanner (MagnetomAera, Siemens), consisting of two sequential spoiled gradient echo (GRE) acquisitions: a reference (Ref) and an MT-weighted image (MT), as seen in Figure 1. Each acquisition is performed with a Cartesian spiral-order trajectory [6] and preceded by a 2D image navigator (iNAV) [7] for translational motion correction. An MT-Ratio (MTR) map is then calculated as: MTR = 100*(Ref-MT)/Ref. Non-rigid motion correction [8] is applied to the coronary vein datasets to improve vessel delineation.
MTR is a composite measure of MT effect and depends on multiple factors, including the Bound Pool Fraction (BPF), exchange and relaxation rates. Bloch simulations using Portnoy’s pulsed MT model [9] were performed to find MT parameters which optimize the sequence sensitivity to collagen content in the myocardium. Simulations results were validated in 10 healthy subjects. An optimized pre-contrast imaging protocol (P1) was subsequently used in 4 patients with chronic scar, undergoing clinical routine cardiac MR with LGE. MT pulse parameters included: Sinc shape, ΔF=3000Hz, repetitions n=20, flip angle=800°, length=20.48ms, delay=1.5ms. Other imaging parameters included: coronal orientation, FoV=300x300mm2, resolution=1.4x1.4x4mm3, GRE readout (TR/TE=3.8/1.6, FA=15°, BW=401Hz/px), bSSFP readout (TR/TE=3.2/1.4, FA=70°, BW=925Hz/px).
Results
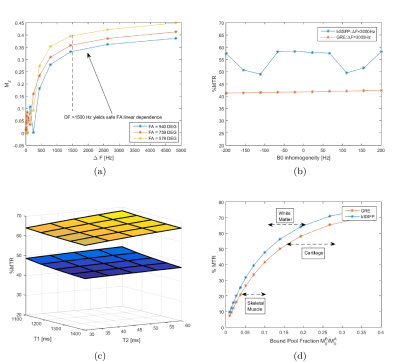
Simulations suggested that a high value of ΔF (>1500Hz) is needed to remove B0 and B1 sensitivity from the MT saturation (Figure 2a). The bSSFP acquisition was found to cause strong variation on a small range of off-resonances (±200 Hz), as opposed to the GRE acquisition which yielded a flat response (Figure 2b). MTR sensitivity to myocardium (free pool) T1 and T2 was shown to be low for both imaging sequences (Figure 2c). MTR sensitivity to collagen content was evaluated by investigating its dependence on BPF, from a two-pool spin model of MT, showing direct and almost linear relationship in the relevant range (Figure 2d).
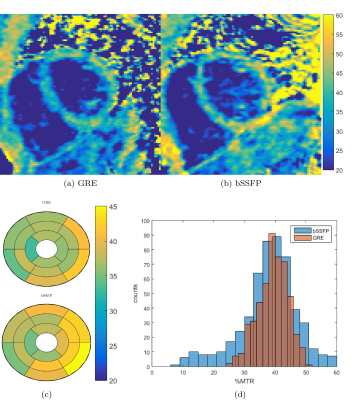
Results in healthy subjects were in accordance with simulation predictions. The bSSFP acquisition yielded higher myocardium-to-blood contrast yet was prone to off-resonance artefacts and the MTR maps were noisier than GRE (see Figure 3a-c). Global myocardium MTR value with bSSFP was MTR=39.2±7.8, while GRE was MTR=37.0±6.0, with a significant reduction in spatial variability, as shown in Figure 3d.
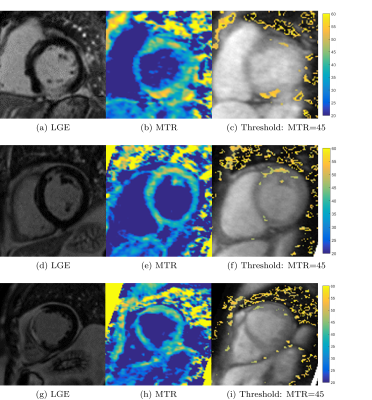
In four patients with prior myocardial infarction, an increase in MTR was observed and visually correlated with the presence of LGE (Figure 4), however a consistent underestimation of the scar area was found. In 5 slices with scar presence, MTR scar area was 29.9±20.2% smaller than LGE, while only in 1 slice was found to be larger (9.7%). No correlation was found for scar located in regions of severe wall-thinning.
In addition, the MT weighted dataset enabled visualisation of detailed cardiac vein anatomy both in patients and healthy subjects (Figure 4).
Discussion
Some limitations found include: (1) decreased MTR towards the apex and regions of wall thinning, possibly due to partial volume effect because of anisotropic spatial resolution; (2) High spatial variation of MTR in myocardium (MTR=37.0±6.0), which might be associated with residual respiratory and cardiac motion, as compared to skeletal muscle (MTR = 41.0±2.4) and liver (MTR=32.4±4.5). This could explain pockets of increased MTR in non-scar regions, Figure 4(c,f,i).Conclusions
We have successfully developed a contrast-free sequence for simultaneous assessment of chronic myocardial scar and cardiac vein anatomy. The proposed MTR maps show agreement with LGE with regard to localization of scar in 4 patients with chronic infarction. Isotropic spatial resolution and a larger cohort is now needed to assess the clinical potential of the technique for myocardial scar detection.
Acknowledgements
This work was supported by the EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1), Siemens Healthcare GmbH and by EPSRC grants EP/P001009/1 and EP/P007619/1.References
[1] McDonald, Robert J., et al. "Intracranial gadolinium deposition after contrast-enhanced MR imaging." Radiology 275.3 (2015): 772-782.
[2] Crooijmans, Hendrikus JA, et al. "Cardiovascular magnetization transfer ratio imaging compared with histology: a postmortem study." Journal of magnetic resonance imaging 40.4 (2014): 915-919.
[3] Weber, O.M., et al. "Assessment of magnetization transfer effects in myocardial tissue using balanced steady state free precession (bSSFP) cine MRI." Magnetic resonance in medicine 62.3 (2009): 699-705.
[4] Stromp, Tori A., et al. "Gadolinium free cardiovascular magnetic resonance with 2-point Cine balanced steady state free precession." Journal of Cardiovascular Magnetic Resonance 17.1 (2015): 1.
[5] Nezafat, Reza, et al. "Coronary magnetic resonance vein imaging: imaging contrast, sequence, and timing." Magnetic resonance in medicine 58.6 (2007): 1196-1206.
[6] Prieto, Claudia, et al. "Highly efficient respiratory motion compensated free‐breathing coronary mra using golden‐step Cartesian acquisition." Journal of Magnetic Resonance Imaging 41.3 (2015): 738-746.
[7] Henningsson, Markus, et al. "Whole‐heart coronary MR angiography with 2D self‐navigated image reconstruction." Magnetic resonance in medicine 67.2 (2012): 437-445.
[8] Cruz, Gastao, et al. "Accelerated motion corrected three‐dimensional abdominal MRI using total variation regularized SENSE reconstruction." Magnetic resonance in medicine 75.4 (2016): 1484-1498.
[9] Portnoy, et al. "Modelling pulsed magnetization transfer." Magnetic resonance in medicine 58.1 (2007): 144-155.
[10] Etienne, Alex, et al. "“Soap‐Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms." Magnetic Resonance in Medicine 48.4 (2002): 658-666.
Figures