4887
An off-resonance correction for in-vivo spiral STEAM diffusion tensor cardiovascular magnetic resonance1Cardiovascular Magnetic Resonance Unit, Royal Brompton Hospital, London, United Kingdom, 2National Heart and Lung Institute, Imperial College, London, United Kingdom, 3National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
Spiral diffusion tensor cardiovascular magnetic resonance demonstrates promising results, but as with all spiral techniques is susceptible to off-resonance artefacts. The majority of off-resonance corrections rely on acquiring a separate field map based on phase differences between acquisitions with different echo times. However, bulk motion is encoded in image phase using STEAM and the motion and off-resonance induced phases are indistinguishable. Here we use a dual spiral approach in each stimulated echo to separate the motion- and off-resonance-induced phase and use this information to correct DT-CMR data.
Introduction:
Spiral diffusion tensor cardiovascular magnetic resonance (DT-CMR) has shown promising initial results (1), however off-resonance may be problematic due to the air-filled lung adjacent to the myocardium. Off-resonance correction typically requires a B0 field map based on phase differences between acquisitions with varying echo times. In stimulated echo acquisition mode (STEAM) DT-CMR bulk motion between the diffusion-encoding gradients is encoded in the image phase, making it impossible to separate motion-induced and off-resonance induced phase. In this work we implement a novel method for acquiring field maps with STEAM DT-CMR acquisitions in the same breath-hold as the DT-CMR data and use these field maps to correct spiral STEAM DT-CMR data.Methods:
In order to separate motion-induced phase from off-resonance induced phase we collect two spirals at the stimulated echo. The first spiral is used to estimate the motion-induced phase which can then be subtracted from the phase of the second spiral leaving only the off-resonance induced phase. By repeating this acquisition with the second spiral delayed from the first, field maps can be calculated.
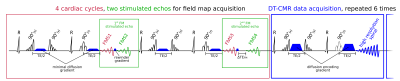
The sequence-diagram is shown in figure 1. In each breath-hold 4 cardiac cycles are used to acquire the field-maps (FM) (2 stimulated echoes) and 12 to acquire the DT-CMR data (6 stimulated echoes). At each of the two FM stimulated echoes two low-resolution spirals are acquired. In both of these stimulated echoes the first spirals (FMS1 and FMS3, see figure 1) start directly at the stimulated echo. Spiral FMS2 starts directly after the first spiral in the first stimulated echo and FMS4 is delayed by ΔTEFM during the second stimulated echo. Motion induced phase was corrected by adding the difference in phase of spirals FMS1 and FMS3 to the phase of spiral FMS4. The field map was calculated from the induced-phase corrected spirals FMS2 and FMS4 (2).
Two off-resonance corrections were tested: During linear field map
interpolation (LFM) (3)
a surface is fitted to the field-map and the fit-parameters are used to correct
the k-space path during gridding. During frequency-segmented reconstruction (FSR)
(4),
each image was reconstructed multiple times with a different constant frequency
offset from -70Hz to +70Hz in steps of 10Hz. The final image was composed by
taking the pixel from the set of frequency offset images with the closest frequency
to that measured at the same pixel in the corresponding field map.
DT-CMR was performed in 3 healthy volunteers age 22-37 on a Siemens 3T
Skyra in a single mid-ventricular slice using the STEAM sequence with
single-shot spirals shown in figure 1.
Spatial resolution was 2.8x2.8x8mm3 for the diffusion encoded
spirals (14ms spiral duration) and 8x8x8mm3 for the FM (3ms spiral
duration). TE=11ms and ΔTEFM=2ms. In each breath-hold the FM data
and 6 diffusion encoding directions were acquired. 10 averages of bmain=600s/mm2
and 2 averages of bref=150s/mm2 were acquired (12 breath
holds). A second order shim was performed with the shim volume chosen to
encompass the left ventricle and minimal additional tissue. In 1 of the 3
subjects the DT-CMR acquisition was repeated with a deliberately poor shim by
setting the shim volume to encompass a much larger region of interest,
including the chest wall, back and shoulders.
The data was reconstructed off-line in MATLAB without an off-resonance
correction, with LFM and FSR. In house MATLAB software (5)
was used to calculate the DT-CMR maps.
Results:
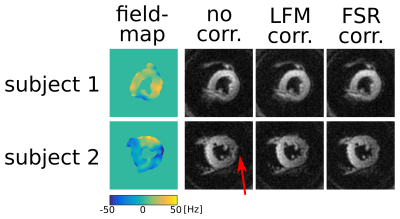
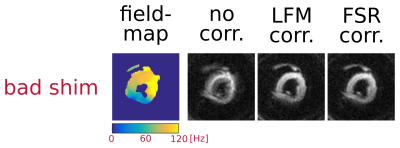
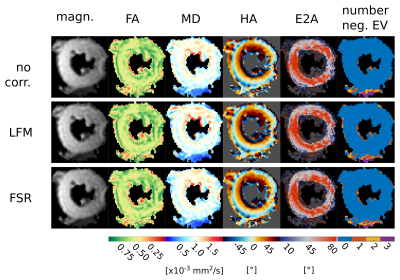
Figure 2 shows an example of field maps and corresponding bref images for two of the volunteers reconstructed without and with both of the off-resonance corrections. Figure 3 shows the field-map and the three reconstructions for a deliberately poorly shimmed acquisition. Figure 4 shows DT-CMR parameter maps for a typical volunteer.Discussion:
This is a successful demonstration of a novel DT-CMR STEAM field-mapping technique with a correction for motion-induced phase. While the left ventricle is a small region that may shim well in some young healthy volunteers, the off-resonance correction results in improvements in image quality particularly in the lateral wall for both diffusion weighted images and DT-CMR maps. In future work, the field map acquisition parameters and correction methods will be optimised and the techniques will be quantitatively evaluated over a larger cohort.Conclusion:
This novel off-resonance correction has shown to provide better quality DT-CMR images particularly in subjects with severe off-resonance artefacts or where the shim performance is poor.Acknowledgements
This work was founded by heart research uk, grant reference number RG2648/15/18.References
1. Gorodezky M, Scott AD, Ferreira P, Nielles-Vallespin S, Khalique Z, Pennell D, Firmin D. In-vivo comparison of STEAM EPI and STEAM spiral diffusion-weighted sequences. International Society for Magnetic Resonance in Medicine Annual Meeting; 2017; Honolulu, USA.
2. Bernstein MA, Grgic M, Brosnan TJ, Pelc NJ. Reconstructions of phase contrast, phased array multicoil data. Magnetic resonance in medicine. 1994;32(3):330-4.
3. Irarrazabal P, Meyer CH, Nishimura DG, Macovski A. Inhomogeneity correction using an estimated linear field map. Magnetic resonance in medicine. 1996;35(2):278-82.
4. Noll DC, Pauly JM, Meyer CH, Nishimura DG, Macovskj A. Deblurring for non‐2D Fourier transform magnetic resonance imaging. Magnetic Resonance in Medicine. 1992;25(2):319-33.
5. Ferreira PF, Kilner PJ, McGill L-A, Nielles-Vallespin S, Scott AD, Ho SY, et al. In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy. Journal of Cardiovascular Magnetic Resonance. 2014;16(1):1.
Figures