4882
A myocardial strain phantom for cardiovascular magnetic resonance1Cardiovascular Magnetic Resonance Unit, Royal Brompton and Harefield Foundation NHS Trust, London, United Kingdom, 2National Heart and Lung Institute, Imperial College London, London, United Kingdom, 3Physics, Imperial College London, London, United Kingdom, 4National Heart Lung and Blood Institute, National Institutes for Health, Bethesda, MD, United States
Synopsis
We have developed a mechanical phantom which replicates myocardial strain in blocks of jelly and sections of myocardial tissue. Initial results show the effects of strain during the diffusion time in diffusion tensor cardiovascular magnetic resonance using STEAM and the use of the phantom evaluating the DENSE strain measurement technique.
Background
Microstructural measures obtained by diffusion tensor cardiovascular magnetic resonance (DT-CMR) with stimulated echo acquisition mode (STEAM) are sensitive to myocardial strain during the diffusion time[1]. Models of this effect consider the myocardium as a jelly-like material[2], but recent studies have questioned the applicability of these models in anisotropic materials like the myocardium[3,4]. We have recently demonstrated a good correspondence between porcine DT-CMR results acquired in-vivo, in-situ arrested, ex-vivo and using histology[5]. However, differences in ventricular loading conditions between the pre- and post-mortem studies confound comparisons. Here we design and test a mechanical CMR compatible phantom which can be used to assess the effects of strain on CMR data and allows a co-registered comparison of DT-CMR in myocardial tissue with and without cyclical strain effects.Methods
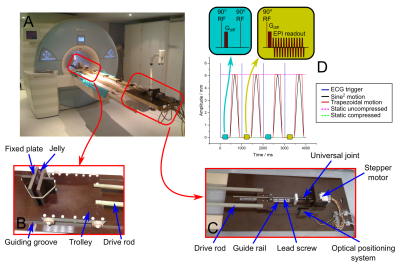
A respiratory motion phantom[6] was modified (figure 1) to cyclically compress slabs of tissue with variable motion trajectories and amplitudes. The phantom consists of a trolley in the scanner bore and a fixed plate. The phantom compresses a slab of tissue between the trolley and the plate and is driven by a microstepping motor and lead screw assembly. The metallic stepper motor and lead screw are separated from the bore via 2.3m plastic rods. Arbitrary motion trajectories are programmed into the microprocessor controller (Taranis, Smartdrive UK). The microprocessor was programmed to produce trapezoidal and a more physiological sine-squared compression (figure 1), with amplitudes 4-8mm and 1s period. Both traces included a static duration before and after the motion. The microprocessor also provided a signal at the beginning of each period, which was used to simulate an ECG and trigger the scanner. A jelly (Rowntrees UK) ~100x100x13mm3 and later a section of mid-myocardial ventricular septum (~70x50x13mm3) from a fresh lamb heart obtained from a butcher’s shop, ventricular weight ~200g, were used as test objects. Imaging was performed at 3T (Siemens Skyra) using a flexible surface coil, placed on top of the test object. DT-CMR was performed using monopolar STEAM-EPI, 6 diffusion directions and b=500smm-2, while the phantom was static in both the relaxed and compressed positions. The acquisition was then repeated whilst the phantom was moving cyclically, with data acquisition triggered to compressed, relaxed and intermediate states. Compression was transmural, along the shortest dimension of the test object and the imaging plane was short-axis-like. Spiral cine displacement encoding with stimulate echoes (DENSE)[7] was also acquired to measure strain in the test objects. DT-CMR data was processed using in-house MATLAB tools[3] and parameters including mean diffusivity (MD), fractional anisotropy (FA) and tensor mode[8] were calculated. Absolute elevation angle was calculated as a surrogate for helix angle due to the differences in geometry between the test object and a heart. Strain information was extracted from the DENSE data using the DENSEanalysis tool from the University of Virginia[9,10].Results
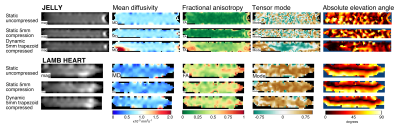
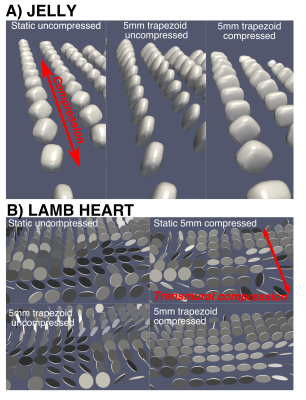
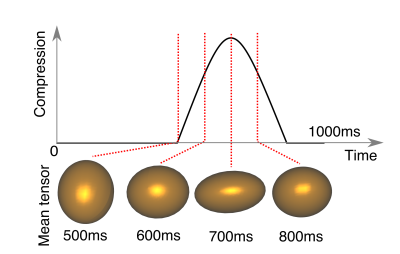
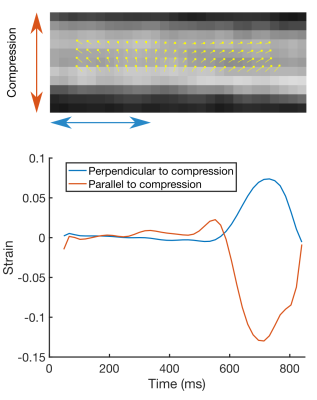
Figure 2 shows example DT-CMR parameter maps for the jelly and lamb septum whilst static and moving cyclically while the water molecules diffuse. The parameter maps appear similar between the uncompressed and compressed static states in both the jelly and the lamb heart, but demonstrate a clear increase in FA, greater coherence in tensor mode and absolute elevation angle in the jelly with the effects of strain. Figure 3 compares the diffusion tensor in jelly and the sheetlet plane (1st and 2nd eigenvectors) between equivalent static and moving acquisitions. In the jelly, the tensor glyphs are elongated along the direction of compression and compressed in the perpendicular direction in the data acquired during trapezoidal motion in the uncompressed state (Figure 5A, central panel) and the opposite is true in the moving compressed acquisition. For the lamb septum, the glyphs rotate between the relaxed and contracted states for both the static and dynamic acquisitions. Figure 4 shows the distortion of the diffusion tensor in jelly caused by sine2 motion when acquired at various stages of compression. Figure 5 shows pixel displacements and mean strain curves obtained using in the phantom and the sine2 trajectory. As the phantom is compressed, tissue stretches in the directions perpendicular to the compression.Conclusion
We have developed a phantom able to simulate myocardial contraction in order to directly assess the contribution of strain to DT-CMR data acquired using STEAM. Despite the absence of active cardiomyocyte contraction these initial results suggest that the sheetlets rotate when the myocardium is compressed. The phantom can also be used to assess the performance of other sequences in the presence of strain or in assessing the performance of techniques used to measure strain and we have demonstrated initial results using DENSE.Acknowledgements
We would like to thank Robin Hardie and the Clinical Engineering Department at the Royal Brompton Hospital for their assistance in the construction of the phantom.References
1. Reese
MRM 1995.DOI:10.1002/mrm.1910340603
2. Reese
JMR-B 1996.DOI: 10.1006/jmrb.1996.0139
3. Ferreira
JCMR 2014.DOI:10.1186/1532-429X-16-S1-P3388
4. Ferreira
MRM 2017.DOI:10.1002/mrm.26850
5. Nielles-Vallespin
JACC 2017. DOI:10.1016/j.jacc.2016.11.051
6.
Scott JMRI 2011.DOI:10.1016/j.mri.2010.11.004
7. Zhong
MRM 2010.DOI:10.1002/mrm.22503
8.
Ennis
MRM 2006. DOI:10.1002/mrm.20741
9.
Spottiswoode
MRM 2007.DOI: 10.1109/TMI.2006.884215
10. Gilliam
et al. 2016. DENSEanalysis. Retrieved from https://github.com/denseanalysis/denseanalysis
Figures