4871
Accelerated free breathing 3D cardiac T1ρ mapping in humans using compressed sensing and improved k-space reordering1Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 2Department of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Cardiac T1ρ MRI is a parametric mapping technique for imaging myocardial fibrosis and may benefit heart disease patients unable to receive contrast agents due to poor kidney function. The purpose of this work was to develop accelerated free-breathing T1ρ mapping MRI that achieves whole heart coverage with superior spatial resolution compared to 2D methods in a reasonable scan time using compressed sensing (CS). The overall hypothesis is that 3-fold accelerated free breathing 3D cardiac T1ρ MRI is feasible.
Introduction
Cardiac T1ρ MRI is a parametric mapping technique for imaging myocardial fibrosis. Since T1ρ MRI is an endogenous contrast technique, it may benefit heart disease patients unable to receive contrast agents due to poor kidney function. Currently used cardiac T1ρ methods have limited spatial coverage and resolution for mapping myocardial disease. This limits the detection of heterogeneous scarred myocardial injury [1,2]. Moreover, the prolonged breath-holds increases patient discomfort and causes misalignment of images. Our aim was to develop a compressed sensing [3] based accelerated free-breathing T1ρ mapping technique that achieves whole heart coverage with superior spatial resolution compared to 2D methods in a reasonable scan time. A novel combination of Split Bregman (SB) [3] based variable substitution and Fourier minimization was used to rapidly minimize the multicoil 3D total variation (TV) [3] cost functional. In addition, a novel k-space reordering technique was used to improve the robustness to motion.Methods
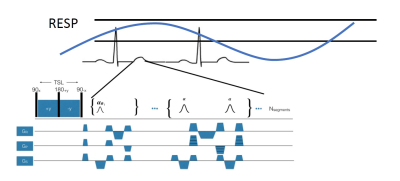
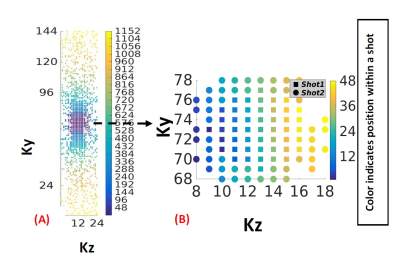
Four prospectively undersampled (R=3) 3D T1ρ datasets were acquired from healthy volunteers on a 1.5 T scanner (Avanto; Siemens) using a T1ρ-prepared balanced steady-state free precession (bSSFP) sequence and a spin echo, spin lock (SL) T1ρ pulse cluster (90x-SLy-180y-SL-y-90-x), B1=500 Hz, and TSL=5-50 ms. Fig 1 shows the proposed 3D T1ρ pulse sequence. The images were acquired at a 1.8x1.8x2 mm3 spatial resolution using a (192×144×24) acquisition matrix. A bell shaped polynomial variable density function was used to undersample the data in ky-kz, while kx was fully sampled. As illustrated in Fig (2), the sampled kspace locations were first sorted based on their radial distance from the kspace center and then divided evenly into the total number of shots. Within each shot, the sampled kspace locations were acquired column-wise. The cost functional used to reconstruct the images is given by: $$ C=\frac{\eta}{2} \sum_ i^N\parallel EC_{i}m-d_{i} \parallel _2^2 +\lambda\mid\triangledown m\mid _{1} $$
Here E is a 3D encoding matrix, that included the sampling pattern and the Fourier operator, C is the coil sensitivity map and k is the measured k-space data and a 3D total variation constraint is used as a regularizer. The cost functional was rapidly minimized using the Split Bregman (SB) variable substitution technique [3], enforcing the following variable substitutions, d=∇m and Pi=Cim. The L2 norm terms of the modified cost functional were minimized using Fourier minimization while the L1 norm terms were minimized using shrinkage [3]. Motion correction using a diffeomorpic registration [4] was performed prior to quantification. The T1ρ maps were generated from the images by fitting a two parameter signal model [1] given by S(t)=S0exp(-TSL/ T1ρ), where S0 is the initial magnetization and TSL is the spin-lock pulse duration.
Results
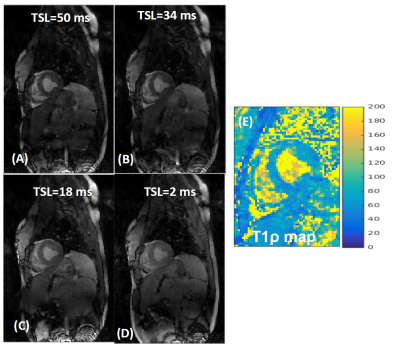
As seen in Fig 3(A)-(D), high quality images were reconstructed from the undersampled k-sapce data using the proposed reconstruction formulation.The myocardial T1ρ map estimated from the undersampled image (Fig 3(E)) is fairly uniform. A comparison with 2D T1ρ is shown in Fig (4). The means T1ρ values from 3D T1ρ and 2D T1ρ were (59.7±4.6) and (63.8±3.8) respectively. The time taken to reconstruct a 3D dataset using the proposed SB based implementation was ~120 sec.Discussion
A free-breathing and cardiac gated 3D T1ρ pulse sequence consisting of a T1ρ preparation period, a magnetization preparation stage and a bSSFP readout was implemented. Data was prospectively acquired during end-expiration using a respiratory navigator. Fig 2(B) shows the k-space ordering for the two shots (acquired over two R-R intervals). When data is acquired over several heartbeats, the proposed reordering ensures that the data collected within an R-R is consistent w.r.t motion. Since most of the low frequency data is acquired is acquired within a single R-R interval (shot1), the amount of motion based artifacts seen in the image is reduced. The T1ρ estimates from the 2D and proposed 3D techniques matched well. This shows that using the proposed data undersampling scheme, the acquisition time could be reduced by a third without the loss of myocardial T1ρ accuracy. The SB based variable substitution formulation presented here uses two variable substitutions, one in the multicoil fidelity constraint and the other in the 3D TV based sparsity constraint. This novel approach to variable substitution allowed for rapid convergence by the use of Fourier minimization and soft thresholding.Conclusion
A highly accelerated, free breathing MRI sequence was developed to obtain high resolution 3D T1ρ maps of the left ventricle. This was achieved by a combination of variable density undersampling (A=3) and 3D TV reconstruction formulation. While further analysis is required, the initial results show that technique could improve non-GBCA myocardial tissue characterization in patients with heart disease.Acknowledgements
This work is supported by R00-HL108157, McCabe Foundation, and W.W. Smith Foundation.References
1. Berisha S, Han J, Shahid M, Han Y, Witschey WRT (2016) Measurement of Myocardial T1ρ with a Motion Corrected, Parametric Mapping Sequence in Humans. PLOS ONE 11(3): Measurement of Myocardial T1ρ with a Motion Corrected, Parametric Mapping Sequence in Humans.
2. Witschey WR, Zsido GA, Koomalsingh K, et al. In vivo chronic myocardial infarction characterization by spin locked cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance. 2012;14(1):37. doi:10.1186/1532-429X-14-37.
3. Goldstein T and Osher S, The Split Bregman Method for L1-Regularized Problems, SIAM Journal on Imaging Sciences 2009 2:2, 323-343
4. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. NeuroImage. 2011;54(3):2033-2044. doi:10.1016/j.neuroimage.2010.09.025.
Figures