4870
Improvement of radiofrequency lesion visualization using 3D T1-weighted compressed sensing imaging1IHU-LIRYC, PESSAC, France, 2Univ. Bordeaux, Centre de recherche Cardio-Thoracique de Bordeaux, Bordeaux, France, 3INSERM U1045, Bordeaux, France, 4Image Guided Therapy, Pessac, France, 5Siemens Healthcare, Saint-Denis, France, 6Bordeaux University Hospital (CHU), Bordeaux, France, 7Siemens Healthcare, Erlangen, Germany
Synopsis
Visualization of acute radiofrequency lesions in the heart is a key point to assess the endpoint of catheter-based anti-arrhythmic therapy. Albeit 3D navigated T1-weighted sequences have proven there reliability to delineate lesion cores and edema, these sequences remain too lengthy/insufficiently spatially resolved to be used clinically. In this study we investigated the benefit of combining 3D T1-weighted acquisition with compressed sensing acceleration to reduce acquisition duration while maintaining sufficient spatial resolution to visualize the core of the lesion and surrounding edema. Methods are evaluated with/without gadolinium injection with different inversion times in vivo in the heart of swine.
Introduction
Catheter-based radiofrequency ablation (RFA) of pathological cardiac substrate is widely used clinically for the treatment of cardiac arrhythmias. Several RFA are usually induced with the objective of creating a geometrical pattern that electrically isolates pathologic tissue from healthy tissue. The procedure is usually performed under X-ray fluoroscopy that provides very limited information on the cardiac substrate. Thus, contact electrical recording is used to verify effectiveness of electrical isolation. However, due to transient inflammatory processes inherent to thermal ablation, unwanted abnormal electrical pathways often reappear after several weeks requiring expensive redo procedures. Thus, there is an important need for 3D, highly spatially resolved, imaging of acute thermal lesions in order to complement the procedure with additional RFA during the initial session. In this study, we propose to validate a 3D T1-weigthed compressed sensing imaging with and without contrast agent injection to visualize acute RFA lesions in the left ventricle (LV) of a pig. Using such an approach, a fully MR-guided RFA for the treatment of cardiac electrical diseases could be envisioned, interleaving real-time cardiac MR thermometry during each RFA with immediate assessment of resulting lesion with the proposed sequence [1], [2].Methods
One swine underwent 8 LV RFA (power and duration ranging [15-25] W and [10-20] s, respectively) under conventional X-ray guidance. This procedure was followed by MR imaging for acute lesion assessment in a 1.5 T scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). An ECG-triggered, crossed-pair-navigated, 3D prototype gradient echo sequence integrating a preparatory inversion pulse was performed. The acquisition was undersampled and its sparsity was enhanced by acquiring k-space lines following a variable-density spiral phyllotaxis pattern in the phase-slice-encoding plane [3]. Image reconstruction was performed using compressed sensing with redundant Haar wavelet regularization [4], combined with an eigenvalue approach for coil sensitivity estimation (ESPIRIT [5]). Sequence parameters were: TR/TE/FA = 6 ms /2.3 ms/20 °, pixel size = 1.3x1.3x1.3 mm$$$^3$$$, BW = 240 Hz/pixel, 96 slices (whole heart), acceleration factor 5.4. For image comparison with the conventional acquisition method, the same sequence parameters were employed. A TI of 700 ms (2 RR intervals) was employed without contrast agent injection. An additional acquisition at 15 minutes post-injection was performed with a TI of 310 ms (over 1 RR).Results
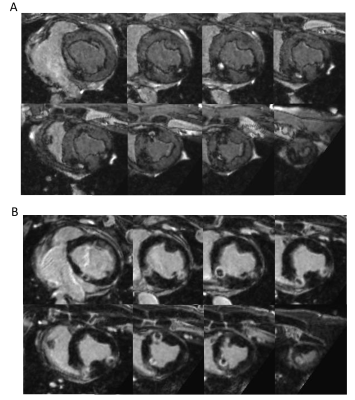
As compared to conventional T1-weighted imaging, the tested sequence parametrization allowed reducing the acquisition time by nearly two folds (45 vs 26 minutes) for identical experimental conditions (85-95 bpm heart rates and 44 to 52% navigator acceptance rate). As compared to a simple zero-filled Fast Fourier Transform (see Figure 1), the proposed algorithm of reconstruction allowed important improvement of image quality and contrast over the entire field of view. A L1 penalization coefficient of the redundant Haar wavelet of 0.0013 was found to be a good compromise between lesion visualization and residual sub-sampling artifacts. The image comparison between the conventional (Figure 2A) and the proposed sequence (Figure 2B) showed a clear improvement of the contrast–to-noise ratio. Using the proposed method, all thermal lesions could be identified both without and with contrast agent injection (see Figure 3). In non-contrast imaging, the contrast of the lesion cores was enhanced, surrounded by dark rings attributed to partial lesion, hematoma, and edema. In post-contrast imaging, the lesion cores were found hypointense surrounded by a contrast-enhanced ring.Discussions and conclusions
This study demonstrates the benefits of compressed sensing to reduce acquisition time as compared to conventional sequences while maintaining a sufficient spatial resolution to visualize thermal lesions in the LV. The proposed approach allows delineating the central region with thermal necrosis and surrounding edema that is prone to recurrence of pathologic electrical pathways after several weeks. Therefore, combining this sequence with real-time MR thermometry is expected to increase safety (online visualization of thermal lesion) and efficacy (improvement of electrical isolation of the pathological tissue) of the therapy. Future studies will focus on non-contrast imaging as contrast agent remains problematic since it may interfere with the following RFA (extrusion of the toxic gadolinium ion from the complex resulting from local temperature increase).Acknowledgements
Acknowledgements This work received financial support from the French National Investments for the Future Programs: ANR-10-IAHU-04 (IHU Liryc) and Laboratory of Excellence ANR-10-LABX-57 (TRAIL), and the research programs ANR-11-TecSan-003-01 (TACIT) and Equipex ANR-11-EQPX-0030 (MUSIC).References
[1] S. Toupin et al., ‘Feasibility of real-time MR thermal dose mapping for predicting radiofrequency ablation outcome in the myocardium in vivo’, J. Cardiovasc. Magn. Reson., vol. 19, Jan. 2017.
[2] M. A. Guttman, S. Tao, S. Fink, A. Kolandaivelu, H. R. Halperin, and D. A. Herzka, ‘Non-contrast-enhanced T 1 -weighted MRI of myocardial radiofrequency ablation lesions: Non-Contrast-Enhanced MRI of RF Ablation Lesions’, Magn. Reson. Med., May 2017.
[3] C. Forman, D. Piccini, R. Grimm, J. Hutter, J. Hornegger, and M. O. Zenge, ‘High-resolution 3D whole-heart coronary MRA: a study on the combination of data acquisition in multiple breath-holds and 1D residual respiratory motion compensation’, Magn. Reson. Mater. Phys. Biol. Med., vol. 27, no. 5, pp. 435–443, Oct. 2014.
[4] Jun Liu et al., ‘Dynamic cardiac MRI reconstruction with weighted redundant Haar wavelets’, presented at the International Society for Magnetic Resonance in Medicine2012.
[5] M. Uecker et al., ‘ESPIRiT-an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA’, Magn. Reson. Med., vol. 71, no. 3, pp. 990–1001, Mar. 2014.
Figures