4868
Temporal evaluation of acute myocardial ischemia/reperfusion injury in rats using 7.0T magnetic resonance imaging and APT102 therapeutic effect1West China Hospital, Sichuan University, Chengdu, China, 2Washington University School of Medicine, St. Louis, MO, United States
Synopsis
Intramyocardial hemorrhage(IMH) and microvascualr obstruction(MVO) were the serious injuries in the early myocardial infarction reperfusion period, which were independent predictors of larger infarct size, lower systolic function and the worse prognosis at follow-up. APT102 as a synthetic apyrase of the hunman nucleoside triphosphate diphosphohydrolase-3(CD39L3) can exhibit ADPase activity, prevent thrombotic reocclusion and decrease infarct size without an increased bleeding risk. The purpose of this study was to continuously detect myocardial ischemia/reperfusion injury and APT102 effect in rats by 7.0T MRI.
Background
In patients with acute myocardial infarction, timely myocardial reperfusion therapeutic methods such as thrombolytic therapy and primary percutaneous coronary intervention can effectively salvage viable cardiomyocytes, limit infarct size, improve long-term myocardial function, finally reduce mortality and prevent heart failure. However, the process of myocardial reperfusion can itself concurrently induce additional capillary damage and cardiomyocyte injury, a common phenomenon known as myocardial reperfusion(I/R) injury. Thereinto, Intramyocardial hemorrhage(IMH) and microvascualr obstruction(MVO) were the serious injuries in the early myocardial infarction reperfusion period, which were independent predictors of larger infarct size, lower systolic function and the worse prognosis at follow-up. APT102 as an optimized human apyrase can exhibit ADPase activity, prevent thrombotic reocclusion and decrease infarct size without an increased bleeding risk. The purpose of this study was to continuously detect myocardial ischemia/reperfusion injury and APT102 effect in rats by 7.0T MRI.Methods
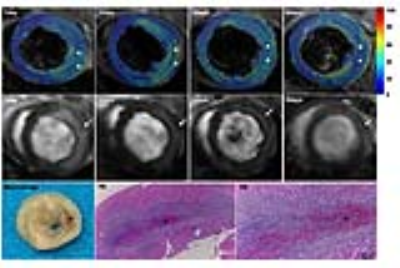
All fourteen Sprague-Dawley rats (female, 200-250g) were underwent the ligation of the left anterior descending coronary artery for 60mins, 10-15mins before reperfusion, APT102(0.3mg/kg) or equal placebo were injected into tail veins. After reperfusion for 1day, 2day, 3day and 5day, rats were injected APT102(0.3mg/kg) and normal saline before scanning and underwent cardiac magnetic resonance at 7.0T MR(Bruker BioSpect70/30,Ettlingen, Germany). T2 mapping with with a fast spin echo technique (scanning parameters: TR/TE=1500ms/10,20,30ms, MTX=192×192, FOV=5cm×5cm, slice thickness=1.5mm) and late gadolinium enhancement (LGE) with a fast imaging with steady precession technique (10minutes after Gd-DTPA injection, scanning parameters: TR/TE=5.2ms/1.8ms, MTX=256×256, FOV=5cm×5cm, slice thickness=1.5mm, slice gap=1.5mm were performed from cardiac base to apex. Images analysis was performed by the custom-made software written in Matlab7.1 and Image J. The epicardial and endocardial outline (papillary muscles was excluded) of left ventricular end-diastole and left ventricular end-systole was manually drawn on cine images slice by slice, and then the left ventricular myocardial volume(LVMV) and left ventricular injection fraction(LVEF) was calculated by multiplying the slice thickness and slice gap. Myocardial edema was defined as a mean signal intensity threshold of 2SDs above the mean remote myocardium and IMH was defined as a hypointense core within hyperintense area on T2 mapping, myocardial infarction was defined as an area with a mean signal intensity at least 5SDs above remote myocardium on LGE. Volume of myocardial infarction, edema and IMH and MVO was expressed as LVMV%. Data were expressed as mean ± SD. All rats were sacrificed after scanning at 5days after reperfusion, myocardial tissue was used for histopathologic staining. RM-ANOVA analysis with and post hoc Bonferroni’ test was used to compare infarct size, edema size, IMH size, LVEF between the control group and APT102 group.Results
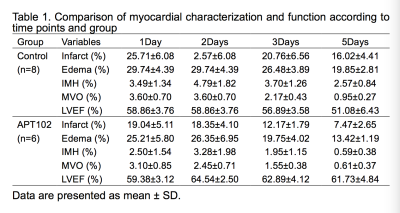
Myocardial ischemia reperfusion model was successfully induced in 14 rats, IMH and MVO has occurred in each rat, shown in Figure 1. The volume of infarction (F=9.556, P=0.009), edema (F=7.048, P=0.021), IMH (F=5.494, P=0.037) and MVO (F=5.167, P=0.042) was significantly lower in APT102 group compared with the control group, whereas LVEF was significantly higher in APT102 group (F=8.989, P=0.011), shown in Table 1.Discussion
Previous studies found that the presence of MVO and IMH was independent predictor of larger infarct size, lower systolic function and the worse prognosis at follow-up, and the presence of IMH generally coupled with MVO. MVO refers to the microvessel injury that prevent adequate myocyte reperfusion despite revascularization and coronary artery reopening, while IMH was considered to be induced by endothelial cells disruption and accumulation of erythrocytes within extracellular space, which is a serve form of MVO. MVO is visualized on magnetic resonance imaging by first-pass perfusion, early gadolinium enhancement and late gadolinium enhancement. IMH can be detected by T1-weighted imaging, T2-weighted imaging, T2*-weighted imaging, susceptibility weighted imaging. APT102 as a synthetic apyrase of CD39L3 can be used as adjunctive therapy for treatment of myocardial infarction and ischemia/reperfusion injury without an increased bleeding risk. These therapeutic effects of APT102 can be measured by noninvasive MR techniques in vivo.Conclusion
APT102 can significantly reduce infarct size, edema size, IMH size and MVO size, especially on 3days and 5dyas after reperfusion; moreover, APT102 can reserve left ventricular systolic function, continuously assessed by CMR.Acknowledgements
No acknowledgement found.References
1. Keeley, E.C., J.A. Boura, and C.L. Grines, Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. The Lancet, 2006. 367(9510): p. 579-588.
2. Derek M. Yellon, D.J.H., myocardial reperfusion injury. 2007.
3. Frohlich, G.M., et al., Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J, 2013. 34(23): p. 1714-22.
4. David J Hearse, A.T., Free Radials and Reperfusion-Induced Arrhythmia: Protection by Spin Trap Agent PBN in the Rat Heart. 1998.
5. Robbers, L.F., et al., Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J, 2013. 34(30): p. 2346-53.
6. Ding, S., et al., Impact of Early ST-Segment Changes on Cardiac Magnetic Resonance-Verified Intramyocardial Haemorrhage and Microvascular Obstruction in ST-Elevation Myocardial Infarction Patients. Medicine (Baltimore), 2015. 94(35): p. e1438.
7. Ye, Y.X., et al., Monitoring of monocyte recruitment in reperfused myocardial infarction with intramyocardial hemorrhage and microvascular obstruction by combined fluorine 19 and proton cardiac magnetic resonance imaging. Circulation, 2013. 128(17): p. 1878-88.
8. Hansen, E.S., et al., Cardiovascular MR T2-STIR imaging does not discriminate between intramyocardial haemorrhage and microvascular obstruction during the subacute phase of a reperfused myocardial infarction. Open Heart, 2016. 3(1): p. e000346.
9. Bulluck, H., et al., Residual Myocardial Iron Following Intramyocardial Hemorrhage During the Convalescent Phase of Reperfused ST-Segment-Elevation Myocardial Infarction and Adverse Left Ventricular Remodeling. Circ Cardiovasc Imaging, 2016. 9(10).
Figures