4867
Single Acquisition Multiparametric MRI for Differentiation of Left Ventricular Scar Tissue Composition1Robarts Research Institute, Western University, London, ON, Canada, 2Graduate Program in Biomedical Engineering, Western University, London, ON, Canada, 3Department of Medicine, Western University, London, ON, Canada, 4Biomedical Engineering, Western University, London, ON, Canada
Synopsis
Cardiac MRI plays a key role in identifying heart failure patients' response to cardiac resynchronization therapy. The identification of healthy myocardial regions for lead implantation is accomplished by means of late gadolinium enhancement MRI using exogenous contrast agents. Most MRI techniques that enable non-contrast cardiac tissue characterization are magnitude-based. MRI phase is an effective standalone source of information. However, most successful phase-based imaging is performed at lower field strength to alleviate field inhomogeneity-related artifacts. We present the application of a robust multiparametric magnitude- and phase-based myocardial mapping approach for scar tissue characterization at 3T.
Introduction
Magnetic resonance imaging has been shown to predict the outcome of interventional procedures such as cardiac resynchronization therapy in heart failure (HF).1 This is accomplished by pre-operative late gadolinium enhancement MRI (LGE-MRI), where the region(s) of myocardial scar are identified and avoided during device implantation. Histological studies2 have demonstrated that fat can be present in chronic scar; using MRI, fat has been visualized within scar (at 1.5T) using acquisitions with and without fat suppression,3 or using the three-point Dixon approach.4 Performing phase-based cardiac MRI at lower field is particularly favoured as there is less concern about susceptibility artifacts at air/tissue interfaces. However, imaging at higher field strength allows for acquisition with higher signal-to-noise ratio among other advantages. In this study we present a robust complex MR data processing approach that facilitates scar characterization through the generation of quantitative multiparametric maps of the heart from a single multi-echo GRE acquisition; results from HF patients are presented and compared to LGE-MRI as the standard.Methods
Image Acquisition: The study was approved by our institutional ethics board. Written informed consent was obtained from all patients. Five HF patients were recruited for this study. Four patients had history of myocardial infarct (MI) (one within 6 months, two within one year, one within 11 years of the scan). The fifth patient did not have history of MI. Multi-slice, multi-echo GRE images of the patients were acquired with the following parameters: TR/TE/ESP: 940/2.23/1.27 ms, in-plane resolution: 1.5 × 1.5 mm2, slice thickness: 6.0 mm, FA: 20°, BW: 1150 Hz/px, GRAPPA: 2. Following this protocol, LGE-MR images were acquired with 8-mm slice thickness 6 minutes after the intravenous injection of contrast. Other imaging parameters included: in-plane resolution: 2.0 x 2.0 mm2, TE/TI/trigger time: 1.02/ 300/617.5 ms.
Post Processing: The complex channel data were saved for offline processing. After phase error correction for bipolar acquisition and removal of the channel-dependent phase term, each channel phase image was unwrapped and subsequently filtered (using a 10-by-10 averaging filter) to calculate an initial B0-map. This B0-map was corrected using a phase error term computed through by matching water- and fat-based fat maps.5 The Additional output of this approach includes a fat fraction (FF) map and R2*-map. Local frequency shift (LFS) maps of the myocardium were then reconstructed for each channel (j) by removing the background field and the effect of chemical shift from the initial complex signal6:
LFSj = (Sodd*e(-ΦcsΔTE) e(-2πfb0ΔTE) )/2πΔTE
where Sodd* is the complex conjugate of signal at odd echo times, ΔTE =TE3-TE1, $Φcs$ is the phase offset due to chemical shift calculated using the fat fraction map, and $fbo$ is the filtered field map.
Once multiparametric maps (B0-, FF-, R2*-, and LFS-maps) were generated for each receiver, channel combination was performed using the magnitude images for weighting.
Analysis: The multiparametric maps were reviewed in reference to LGE-MRI for each participant by an experienced imaging researcher and a cardiologist. The correspondence between the scar locations on each data was noted.
Results
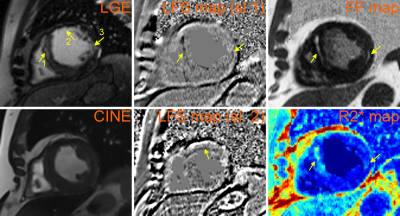
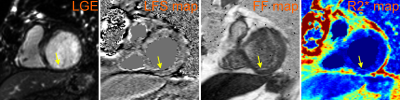
The multiparametric maps were successfully generated for all patients. The two patients with history of MI within a year of scan demonstrated scar tissue in the intramyocardial and subendocardial regions. Figures 1 and 2 demonstrate these scar regions on LGE-MRI and the matched multiparametric R2*-, FF- and LFS-maps. Figure 1 additionally shows a matched CINE image, which, together with the LGE, demonstrates the extent of myocardial scar. Two consecutive slices of LFS map are shown to enable the visualization of all three scar regions pointed to in Figure 1.
The individual quantitative maps clearly depict unique information about the structures within the scar; the R2* map depicted the global region of the myocardial wall affected by Mthe infarct while the phase-based masks depicted the fatty infiltration (on the FF-maps) and the extracellular collagen infiltration within the myocardium (on the LFS-maps).
Discussion and Conclusion
We demonstrated the ability to extract multiparametric maps of the heart using a single readily available multi-echo GRE acquisition at 3T. Myocardial quantitative mapping was made possible using a processing approach applied to the individual channels prior to the application of channel combination. Careful phase error correction should be implemented to account for channel-specific phase bias as well as other phase errors (i.e. bipolar error correction).
The presented approach does not require external contrast agents, which is a major benefit for patients with poor renal function. However, a larger clinical trial is needed to validate the ability of this approach to potentially replace LGE-MRI for patients who cannot tolerate contrast injection.
Acknowledgements
The authors acknowledge the help of the research coordinators at London Health Sciences for their help with patient recruitment.References
[1] White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, Klein G, Drangova M. Delayedenhancement magnetic resonance imaging predicts response to cardiacresynchronization therapy in patients with intraventricular dyssynchrony. J Am CollCardiol 2006;48(10):1953-1960.
[2] Baroldi G, Silver MD, De Maria R, Parodi O, Pellegrini A. Lipomatous metaplasia inleft ventricular scar. The Canadian journal of cardiology 1997;13(1):65-71.
[3] Mordi I, Radjenovic A, Stanton T, Gardner RS, McPhaden A, Carrick D, Berry C,Tzemos N. Prevalence and Prognostic Significance of Lipomatous Metaplasia inPatients With Prior Myocardial Infarction. JACC Cardiovasc Imaging2015;8(9):1111-1112.
[4] Goldfarb, James W., Marguerite Roth, and Jing Han. "Myocardial fat deposition after left ventricular myocardial infarction: assessment by using MR water-fat separation imaging." Radiology 253.1 (2009): 65-73.
[5] Hosseini, Z., Liu, J., Drangova, M. "Quantitative Cardiac B0, Fat Fraction, and R2* Mapping Using Pre-Channel-Combination Phase Processing." in Proceedings of International Magnetic Resonance in Medicine, 2017 Honolulu, USA.
[6] Liu, Junmin, Goldfarb, James W., and Maria Drangova. "Myocardial Local Frequency Shift Mapping." in Proceedings of International Magnetic Resonance in Medicine, 2016, Singapore.
Figures