4865
23Na MRI at 7 Tesla for Early Response Assessment in Patients with Glioblastoma and Skull Base Meningioma1Department of Radiation Oncology, University Hospital of Heidelberg, Heidelberg, Germany, 2Clinical Cooperation Unit Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Division of Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 5Institute of Radiology, University Hospital Erlangen, Erlangen, Germany
Synopsis
Radiotherapy is a cornerstone in the treatment of glioblastoma and skull base meningioma. Here, the first results of a prospective longitudinal study employing 23Na MRI for the response evaluation of glioblastoma and skull base meningioma patients during radiotherapy are presented. The study results show that radiation treatment of glioblastoma leads to considerable changes in sodium concentrations within the tumor and the surrounding edema that are dependent on treatment response.
Introduction
Radiotherapy is a cornerstone in the treatment of glioblastoma and skull base meningioma. At present, the response assessment is mainly based on contrast-enhanced T1- and T2-weighted MRI images acquired at 3 Tesla [1], with well-known limitations. The increasing utilization of ultra-high magnetic fields with enhanced signal-to-noise ratios bears great potential to enhance biofunctional imaging [2, 3]. In this context, sodium MRI is an emerging approach for tumor characterization [4, 5, 6, 7] and response evaluation [8, 9]. Here we present the first results of a prospective longitudinal study employing sodium MRI on a 7-Tesla scanner during radiotherapy of brain tumors.Methods
Three glioblastoma and four meningioma patients underwent imaging on a 7T MRI scanner (Siemens Healthineers, Erlangen, Germany) in addition to the standard 3T MRI protocol before, during, and after definitive treatment. High-resolution T2 TSE and T2 FLAIR imaging was performed using a 24-channel single-resonant (1H) head coil. Sodium MR images were acquired with a double-resonant (1H/23Na) quadrature birdcage coil using an in-house density-adapted 3D radial projection pulse sequence [10] (TR / TE = 160 ms / 0.35 ms, NProjections = 4000, nominal resolution (Δx)3 = (3 mm)3, Tacq = 10:40 min) and an iterative 3D DLCS reconstruction algorithm [11]. The reconstruction parameters were: block size B = 3, dictionary size D = 80, sample number Nsamp = 500,000, and weighting factor for the regularization μ = 0.5. Quantification of the 23Na data was achieved by placing reference tubes (0.3% and 0.6% NaCl) adjacent to the head and correcting for transmit and receive inhomogeneities with a double angle method. 7T and clinical 3T MRI images from different time points were co-registered manually using MITK [12]. ROIs were delineated on clinical standard images by an experienced radiation oncologist: regions with T1-weighted gadolinium contrast enhancement (gdce) representing tumorous tissue, regions of T2 FLAIR hyperintensity representing edema, and normal-appearing white matter (nawm). Response evaluation was done according to RANO criteria [1].Results
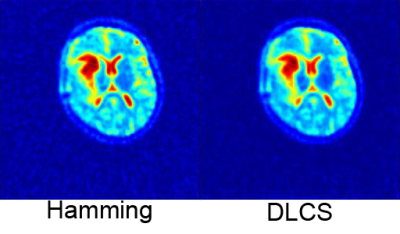
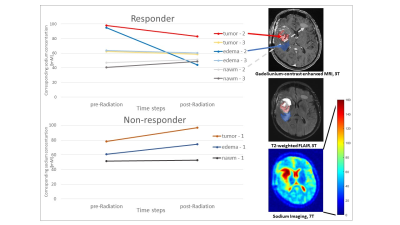
The benefit of iterative reconstruction compared to Hamming filtering is shown in Fig. 1. Noise is reduced, both outside the FOV and within, which enables more accurate quantification of the obtained data. In all glioblastoma patients, clear changes in sodium mean signal intensity was observed in regions of tumorous tissue and edema (Fig. 2). Furthermore, there was a tendency towards decreasing sodium values in tumorous tissue and edema in the two therapy responders, whereas the opposite was true for the single non-responder. This tendency already became obvious in the first examination right after therapy. Conversely, neither the nawm in glioblastoma patients nor the tumorous tissue and nawm in the meningioma cohort (Fig. 3) showed obvious signal changes.Discussion & Conclusion
The stability of sodium signals in non-infiltrative disease suggests indifference of sodium imaging towards possible radiotherapy-induced changes in unaffected white matter. Thus, the signal changes in the glioblastoma cohort might particularly reflect treatment response of high-grade tumor tissue. Radiation of glioblastoma was accompanied by considerable changes of sodium signal within the tumor tissue and surrounding edema already early after therapy, with different trends in treatment responders versus non-responders. Accordingly, sodium MRI might yield early information about treatment response in glioblastoma and merits further investigation.Acknowledgements
No acknowledgement found.References
[1] Murphy, Circ Res 2000; 86(3):326-33
[2] Thouyz et al., Am J Physiol Heart Circ Physiol 2008; 294(3):1103-18
[3] London, Annu Rev Physiol 1991; 53:241-58
[4] Gupta et al, J Biol Chem 1978; 253:6172-176
[5] Gupta et al, Ann Rev Biophys Bioeng 1984; 13:221-46
[6] Ouverkerk et al, Radiology 2003; 227:529-37
[7] Biller et al, Am J Neuroradiol 2015; 37: 66-73
[8] Golding et al, Magn Reson Med 1996; 35(2):174:85
[9] Halverson et al, NMR Biomed; 5:53-58
[10] Chung et al, Chem Phys Lett 1990; 172(1) :94-98
[11] Nagel et al, Magn Reson Med 2009; 62(6):1565-73
[12] Fessler et al, IEEE Trans Signal Process; 2003 ; 51(2):560-74