4863
Late-delayed perfusion decrease following radiochemotherapy in glioblastoma patients1PET Center, Institute of Radiopharmaceutical Cancer Research, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 2Rochester Institute of Technology, Rochester, NY, United States, 3Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 4Department of Radiology, Academic Medical Center, Amsterdam, Netherlands, 5Department of Radiology, University Hospital Carl Gustav Carus, Dresden, Germany, 6University Hospital Bonn, Bonn, Germany, 7Department of Radiation Oncology, University Hospital Carl Gustav Carus, Dresden, Germany, 8OncoRay – National Center for Radiation Research in Oncology, Dresden, Germany, 9German Cancer Consortium (DKTK), Dresden, Germany, 10German Cancer Research Center (DKFZ), Heidelberg, Germany, 11Institute of Radiooncology, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany, 12NCT – National Center for Tumor Disease, Dresden, Germany, 13Department of Nuclear Medicine, University Hospital Carl Gustav Carus, Dresden, Germany
Synopsis
Temozolomide-based radiochemotherapy (RCT) is a treatment standard for glioblastoma patients. However, RCT is associated with risks of neurocognitive decline. Perfusion is a possible early marker of tissue damage and has been shown to correlate with cognitive changes in many diseases. Perfusion decrease at 3 to 6 months after RT was recently reported in glioblastoma patients. However, it remains unclear whether the decrease is reversible and thus possibly a precursor of the late-delayed cognitive changes. In this study, we have measured perfusion changes up to 18 months following RCT. No further progress of perfusion deficits was found indicating that the early perfusion decrease is predictive of late perfusion decrease and might thus be connected with cognitive decline.
Introduction
Radiochemotherapy (RCT) with temozolomide is a standard therapy for glioblastoma patients and can significantly prolong survival1. However, it has been shown that RCT can negatively influence the neurocognitive abilities and may cause structural2,3 and functional4,5 damage in healthy brain tissue. Cerebral blood flow (CBF) decrease has been shown to correlate with cognitive decline in many diseases and therefore can be an early marker of the risk of cognitive deterioration. Recently, a decrease in CBF, measured with arterial spin labeling (ASL) and dynamic susceptibility-weighted (DSC) MRI, was reported at three to six months after RCT4,5. However, it remains unclear whether perfusion normalizes or whether alterations remain irreversible. To this end, in this study, we focused on late-delayed changes in CBF and related arterial transit time (ATT) changes of up to eighteen months after RCT.Methods
Sixty-two patients (mean age 55.0±14.2 years) with unilateral glioblastoma were studied. RCT after the Stupp scheme (6 weeks of RT with 2Gy fractions 5 days/week; concomitant temozolomide 75mg/m2) was delivered to all patients1. Photon therapy using either 3D-conformal (n=25) or intensity-modulated (n=20) RT planning or proton therapy with passive double scattering (n=17) were used. Patients were first scanned after tumor resection before RCT and then on a maximum of six follow-ups in three-months intervals after the end of RCT. On each session, T1-w and pseudo-continuous ASL (pCASL) images were acquired using a 3T Philips Ingenuity PET/MR. pCASL parameters were: 2D-EPI readout, TR/TE 3765/11ms, voxel size 2.75x2.75x6mm3, 17 slices (0.6mm gap), 30 control/label pairs, background suppression, labeling time/delay 1650/1525ms. A reference M0 image was acquired 5000ms after saturation; T1-w: 3D-TFE, voxel size 1x1x1mm3.
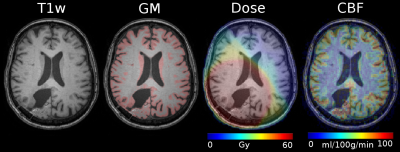
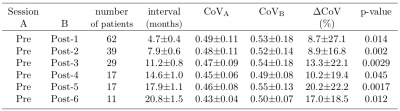
Data were processed using Matlab and SPM12 toolbox. pCASL images were quantified using the single-compartment model6, and co-registered with the T1-w image and the radiation dose map. Sessions with motion, labeling artifacts and ATT artifacts in ASL images were excluded. The T1-w images were segmented to gray (GM) and white matter (WM), see Figure 1. ATT changes were assessed using the spatial coefficient of variation (sCoV) method7 which quantifies vascular artifacts in the CBF maps and evaluates global ATT. Mean GM CBF (for GM>70%), sCoV, and radiation dose in the healthy hemisphere contralateral to the tumor were calculated for each session.
Mean changes in GM CBF and sCoV across sessions were calculated and their significance assessed using a paired t-test at significance level of P=0.05. Changes in spatial CoV were also evaluated in all patients without excluding the ATT artifacts.
Results
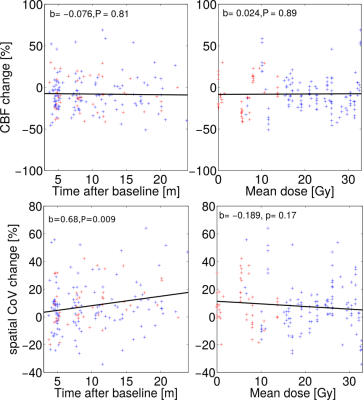
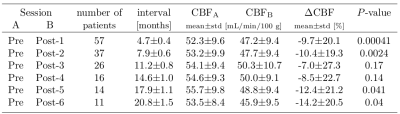
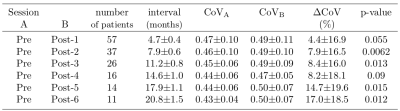
The CBF decrease compared to pre-therapy baseline was 7.0–14.2% and significant on all but post-therapy sessions 3 and 4 (Table 1). There were no significant differences in CBF between the first and later post-therapy sessions. The sCoV increased from baseline by 4.4–17.0%; the increase was significant on all but post-therapy sessions 1 and 4 (Table 2). When all sessions were included, sCoV increased by 8.7–20.2% and the changes were significant on all sessions (Table 3). Compared to the first post-therapy session, sCoV further increased on post-therapy sessions 5 (10.2%, p=0.027) and 6 (9.3%, P=0.011). A multivariate analysis revealed that age affected both CBF (β=-0.32, P=0.017) and sCoV (β=0.23, P=0.025) changes. Time after therapy (β=0.87, P=0.00083) was significant only for sCoV but not CBF (Figure 2). There was no significant effect of dose, sex, BMI or clinical target volume on either sCoV or CBF (Figure 2).Discussion
The results show that the early CBF changes after RCT are not recovered at 18 months after the therapy but also seem to not progress further after the completion of the therapy. Comparable to CBF, sCoV showed ATT increase that could be explained through CBF-decrease related decrease in blood velocity in macrovasculature. There is an indication of further sCoV increase on the last two sessions which was not accompanied by further CBF decrease. This could possibly indicate RT-induced late effects on the macrovasculature but needs to be confirmed in a larger sample size to rule out potential confounding effects. Nevertheless, sCoV was shown to have a higher statistical power to detect similar changes as CBF in the same group of patients. Similar to previous findings4,5, only a minimal effect of mean radiation dose and radiation modality on perfusion changes was observed.Conclusion
The CBF decline can provide an early biomarker and a direct link to the neurocognitive decline, however, this hypothesis needs to be validated with data coupled to neurocognitive tests.Acknowledgements
No acknowledgement found.References
1. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352(10):987–996.
2. Armstrong CL, Gyato K, Awadalla AW, Lustig R, Tochner ZA. A Critical Review of the Clinical Effects of Therapeutic Irradiation Damage to the Brain: The Roots of Controversy. Neuropsychology Review. 2004;14(1):65–86.
3. Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. Journal of Clinical Oncology. 2012;30(30):3675–3686.
4. Petr J, Platzek I, Seidlitz A, Mutsaerts HJMM, Hofheinz F, Schramm G, et al. Early and late effects of radiochemotherapy on cerebral blood flow in glioblastoma patients measured with non-invasive perfusion MRI. Radiotherapy and Oncology. 2016;118(1):24–28.
5. Markus Fahlström, Tufve Nyholm, and Elna-Marie Larsson. Are radiation-induced perfusion changes in normal appearing brain tissue a confounding factor in tumour response evaluation with DSC-MRI? ISMRM 2017.
6. Alsop, D. C., Detre, J.A., Golay, X., Günther, M., Hendrikse, J., Hernandez-Garcia, L., Hanzhang, L., MacIntosh, B.J., Parkes, L. M., Smits, M., van Osch, M. J. P., Wang, D. J. J., Wong, E. C., Zaharchuk, G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine. 2015; 73(1):102-16.
7. HJMM Mutsaerts, J Petr, L Václavů, JW van Dalen, AD Robertson, et al. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. Journal of Cerebral Blood Flow & Metabolism. 2017; in press.
Figures