4861
Volumetric Amide Proton Transfer-Weighted (APTw) Image Metrics as Biomarkers for the Identification of Tumor Progression in Patients with Post-treatment Glioblastoma1Johns Hopkins University, Baltimore, MD, United States, 2Zhujiang Hospital, Southern Medical University, Guangzhou, China, 3Center for Brain Imaging Science and Technology, Department of Biomedical Engineering, Zhejiang University, Hangzhou, China
Synopsis
We quantified the accuracy of volumetric APTw image derived metrics in identifying recurrent malignant glioma. 31 patients with suspected recurrent glioblastoma underwent a volumetric APTw imaging sequence at 3T. Volumes with Gd-enhancing, FLAIR abnormality and APTw hyperintensity were drawn as regions of interest (ROIs). Ratios and APTw histogram parameters of volumetric ROIs were calculated and analyzed. There were significant differences in multiple parameters between treatment effects and recurrent tumor. APT/Gd and Gd-APT10% showed the highest diagnostic performance. FLAIR-Mean showed reasonable diagnostic performance with great operation simplicity.
Target audience
Researchers and clinicians interested in novel diagnostic imaging techniques and brain tumor imaging.Purpose
Post-treatment glioblastomas are biologically complex and often demonstrate highly variable, heterogeneous imaging characteristics. Currently, distinguishing recurrent tumor from treatment effects remains a major clinical challenge, even with improvements in some advanced imaging modalities(1). Thus, post-treatment patients with suspected recurrent tumor are often referred for repeat surgery to obtain pathologic confirmation of active cancer. APTw-MRI is a novel molecular imaging technique that generates image contrast primarily based on endogenous cellular proteins(2-5). Here, we assessed whether volumetric APTw-MRI metrics could identify recurrent tumor in a heterogeneous background in patients with suspected recurrent glioblastoma, and investigated which parameters had feasibility and reproducibility for further radiomic studies.Methods
Subjects: We retrospectively analyzed 31 patients with post-treatment glioblastomas, who underwent chemoradiotherapy after surgery, and subsequently developed Gd-enhancing lesions on Gd-T1w MRI that was presumed to be suspicious for tumor recurrence. Diagnosis was made by the RANO Criteria(1). Pathological results were prior to the RANO Criteria for patients who underwent surgery within two weeks after MRI scanning.
MRI protocol: MRI scans included T2w, FLAIR, Gd-T1w, and volumetric APTw. A fast 3D APTw imaging sequence (RF saturation power=2 μT; saturation time=800 ms; 15 slices; slice thickness=4.4 mm) was used(4). To correct for B0 field inhomogeneity effects, APTw imaging was acquired with a six-offset protocol (±3, ±3.5, ±4 ppm from water), together with a WASSR scan(6). The total scan time was 8 min 6 sec. APTw images were calculated using MTRasym(3.5ppm) analysis(6).
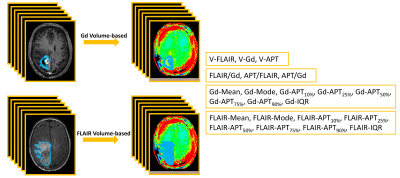
Image analysis: A neuroradiologist and a research scientist draw volumetric ROIs manually separately (Fig. 1). Volumes with Gd-enhancement, abnormal FLAIR intensity, and APTw hyperintensity (≥1.64%, a cut-off APTw value for the differentiation of recurrent tumor from treatment effects, an unpublished result) were recorded as V-Gd, V-FLAIR, and V-APT. Large cysts were excluded when drawing volumetric ROIs with Gd-enhancement, but included for volumetric ROIs with abnormal intensity on FLAIR images. The volumetric ratios were recorded as FLAIR/Gd, APT/FLAIR, and APT/Gd, respectively. Histogram APTw parameters were calculated from the volumetric ROIs of Gd-enhancement and abnormal FLAIR intensity for each patient. The histogram parameters including Mean, Mode, interquartile range (IQR), APTw value percentiles (recorded as Gd-APT10% and FLAIR-APT10%, respectively) from the volumetric ROIs on FLAIR and Gd-T1w images were calculated and recorded (Fig. 1).
Statistical analysis: The inter-rater agreement between the two readers for the assessment of volumetric ROIs was analyzed by using the Intraclass Correlation Coefficient (ICC) and by applying a one-way intraclass correlation coefficient with a random rater assumption. The comparisons of all MRI parameters between treatment effects and recurrent tumor were performed using an independent samples t-test to analyze the statistical differences. The accuracy for the discrimination of treatment effects and recurrent tumor were further determined by a ROC analysis. The alpha level of all tests was set at P < 0.05.
Results and Discussion
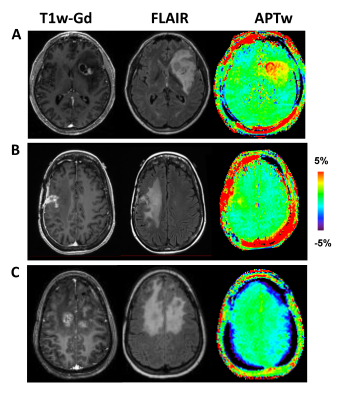
After reviewing the histopathologic analysis on biopsy specimens or longitudinal MRI analysis acquired over more than six months, 20 patients were confirmed as recurrent tumor (Fig. 2A,B), and 11 as treatment effects (Fig. 2C). Inter-rater reliability, as determined by ICC, was acceptable for Gd volume-based ROIs (0.713-0.795), and there was a good consistency between raters with the ICC values of FLAIR volume-based ROIs (0.928-0.984).
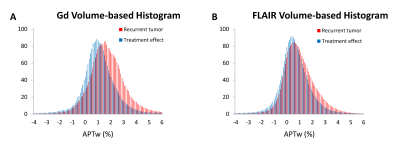
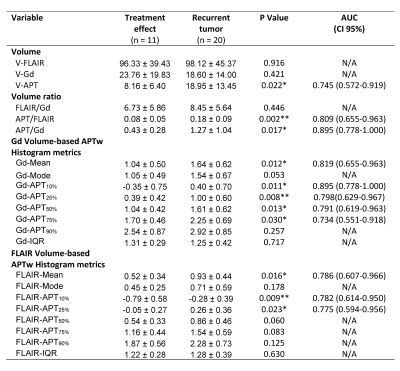
Average histograms of APTw values from recurrent tumors were right shifted, compared with those from treatment effects (Fig. 3), a trend that was obvious for Gd volume-based histograms (Fig. 3A). Quantitatively (Table 1), V-APT, APT/FLAIR, APT/Gd, Gd-Mean, Gd-APT10%, Gd-APT25%, Gd-APT50%, Gd-APT75%, FLAIR-Mean, FLAIR-APT10%, and FLAIR-APT25% showed significantly different between treatment effects and recurrent tumor. For these parameters with statistical difference, further ROC analysis demonstrated that APT/Gd and Gd-APT10% yield the highest AUC (0.895) among the volumetric metrics. The corresponding sensitivity and specificity were 62.2% and 94.2% for APT/Gd, and 71.7% and 90.1% for Gd-APT10%, respectively, with cutoff values of APT/Gd > 0.54 and Gd-APT10% ≥ 2.15% that predicted tumor progression in the scenario of suspicious, but not definitive recurrence.
The volumetric FLAIR volume-based APTw parameters show the acceptable diagnostic performance (FLAIR-Mean shows the highest AUC with 0.786 among the volumetric FLAIR parameters), they also showed the great potential for automatic tumor segmentation due to the operation simplicity (volumetric ROIs of FLAIR cover the contour of lesion with abnormal FLAIR intensity including necrosis, vessels, and hemorrhages) and great reproducibility, which would be easily translated to radiomic features for machine learning in the near future.
Conclusions
The current study suggests that APTw image-derived metrics are valuable biomarkers of tumor recurrence in patients treated with standard chemoradiation. The quantitative APTw metrics would be applied as radiomic features for future machine-learning-study in patients with post-treatment glioblastomas.Acknowledgements
No acknowledgement found.References
1. Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, DeGroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963-1972.
2. Zhou J, Zhu H, Lim M, Blair L, Quinones-Hinojosa A, Messina SA, Eberhart CG, Pomper MG, Laterra J, Barker PB, van Zijl PC, Blakeley JO. Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging 2013;38:1119-1128.
3. Togao O, Keupp J, Hiwatashi A, Yamashita K, Kikuchi K, Yoneyama M, Honda H. Amide Proton Transfer Imaging of Brain Tumors Using a Self-Corrected 3D Fast Spin-Echo Dixon Method: Comparison With Separate B-0 Correction. Magnet Reson Med 2017;77:2272-2279.
4. Jiang SS, Eberhart CG, Zhang Y, Heo HY, Wen ZB, Blair L, Qin HM, Lim M, Quinones-Hinojosa A, Weingart JD, Barker PB, Pomper MG, Laterra J, van Zijl PCM, Blakeley JO, Zhou JY. Amide proton transfer-weighted magnetic resonance image-guided stereotactic biopsy in patients with newly diagnosed gliomas. Eur J Cancer 2017;83:9-18.
5. Park JE, Kim HS, Park KJ, Kim SJ, Kim JH, Smith SA. Pre- and posttreatment glioma: Comparison of amide proton transfer imaging with MR spectroscopy for biomarkers of tumor proliferation. Radiology 2016;278:514-523.
6. Zhou J, Payen J, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature Med 2003;9:1085-1090.
Figures