4856
Combined Diffusion Kurtosis Imaging and Dynamic Susceptibility Contrast Magnetic Resonance Perfusion Imaging for In Vivo Molecular Profiling of Human Glioma.1Neuroradiology, Eberhard Karls University Tübingen, Tübingen, Germany, 2Neuropathology, Eberhard Karls University Tübingen, Tübingen, Germany, 3Neurology, Eberhard Karls University Tübingen, Tübingen, Germany, 4Neurosurgery, Eberhard Karls University Tübingen, Tübingen, Germany, 5Preclinical Imaging; Nuclear Medicine, Eberhard Karls University Tübingen, Tübingen, Germany
Synopsis
The purpose of this study was to assess the diagnostic performance of combined DKI and DSC-MRI maps for in vivo assessment of the 2016 WHO integrated glioma grades.
Histogram parameters of DKI show a higher diagnostic performance than those of DSC-MRI in stratifying gliomas according to the integrated molecular approach of 2016 CNS WHO. However, DSC-MRI may provide additional insight into the MGMT methylation profile of primary IDH wild-type GBM. Thus, combined DKI and DSC-MRI provide promising potential biomarkers for glioma.
Introduction
Accurately identifying and grading glioma is important to determine clinical management and the prognosis [1, 2]. Histopathologic examination is essential for definitive grading and subsequent molecular stratification of glioma [3, 4]. However, robust and reliable non-invasive tumor grading is needed for follow-up of suspected low-grade glioma, for optimal care of patients who are not eligible for surgery, for those with an increased risk of post-biopsy complications, or for those being monitored for potential tumor recurrence [5].
The recently updated World Health Organization classification (revised 4th edition) of tumors of the central nervous system (2016 CNS WHO) combines both histopathological and molecular features into an “integrated diagnosis” [1]. The molecular stratification is essential for estimating individual prognosis [6, 7]. The relevant molecular characteristics are isocitrate-dehydrogenase (IDH) 1/2 mutation status and chromosome 1p/19q loss of heterozygosity (LOH). They are complemented by alpha-thalassemia/mental retardation syndrome X-linked (ATRX) expression resulting in an alternative lengthening of telomeres (ALT) phenotype [8]. The ATRX status itself confers a prognostic potential in diffuse gliomas [9]. O6-methylguanine DNA methyltransferase (MGMT) can be regarded as an independent prognostic factor in patients with primary glioblastoma (GBM) [10].
Diffusional kurtosis imaging (DKI) as an extension of the diffusion tensor imaging (DTI) method allows for simultaneous direction-dependent estimates of the apparent diffusion coefficient (ADC) and apparent kurtosis coefficient AKC [11]. DKI reflects the heterogeneity and microstructural architecture of a biological tissue such a brain tissue because diffusion barriers alter the water diffusion probability distribution [12, 13].
Dynamic susceptibility-weighted magnetic resonance perfusion imaging (DSC-MRI) is an established imaging method that allows calculation of tumor tissue perfusion [14]. Cerebral blood volume (CBV) results from DSC-MRI reflect the vascular proliferation in tumors [15, 16].
Purpose
To assess the diagnostic performance of combined DKI and DSC-MRI maps for in vivo assessment of the 2016 World Health Organization Classification of Tumors of the Central Nervous System (2016 CNS WHO) integrated glioma grades.Methods
One hundred patients
with histopathologically-confirmed glioma who provided written informed consent
were retrospectively assessed between 01/2014 and 03/2017 from a prospective
trial approved by the local institutional review board. Ten histogram
parameters of mean kurtosis (MK) and mean diffusivity (MD) metrics from DKI as
well as normalized relative CBV (rCBV) values from DSC-MRI were independently
assessed by two blinded physicians from a volume of interest around the entire
solid tumor. One-way ANOVA on ranks (Kruskal-Wallis test) with post-hoc
Dunn-Bonferroni correction was used to compare MK, MD, and rCBV histogram
parameter values between 2016 CNS WHO-based histological findings and molecular
characteristics. Receiver operating characteristic analysis was performed on MK,
MD, and rCBV histogram parameters for significant results. A
classification and regression tree (CART) algorithm with 10-fold
cross-validation was used to calculate the diagnostic accuracy.Results
For DKI, the 25th, 50th, 75th, and 90th percentiles of MK and average MK showed significant differences between IDH1/2wild-type gliomas, IDH1/2mutant gliomas, and oligodendrogliomas (OD1p/19qLOH) with synchronous chromosome 1p/19q loss of heterozygosity and IDH1/2mutation (Fig. 1; p value range, <0.001-0.032). The 50th, 75th, and 90th percentiles showed a slightly higher diagnostic performance (area under the curve (AUC) range, 0.868-0.991) than average MK (AUC range, 0.855-0.988) in classifying glioma according to their molecular profile.
For DSC-MRI, the 25th, 50th, and 75th percentiles of rCBV and average rCBV showed significant differences between IDH1/2mutant and IDH1/2wild-type gliomas (p<0.0001) as well as between IDH1/2mutant gliomas and OD1p/19qLOH (Fig. 2; p<0.0001). However, there is substantial overlap between IDH1/2wild-type gliomas and OD1p/19qLOH (p=1.0). Nonetheless, the 25th, 50th, and 75th percentiles and the SD of rCBV as well as average rCBV were significantly higher in IDHwild-type gliomas with methylated O6-methylguanine DNA methyltransferase (p value range, 0.004-0.021; AUC range, 0.742-0.757) than in those with unmethylated MGMT. The 75th percentile of rCBV correctly predicted MGMT methylation status in 79.5% of all primary IDHwild-type GBMs. We found no significant differences of MK, MD, and rCBV values among astrocytomas grade II, grade III, and GBM both within the IDHmutant and the IDHwild-type molecular group. Additionally, for OD1p/19q-LOH, we found similar MK, MD, and rCBV values among oligodendroglioma grade II and III (Fig. 3 and 4).
Conclusions
Histogram parameters of DKI show a higher diagnostic performance than those of DSC-MRI in stratifying gliomas according to the integrated molecular approach of 2016 CNS WHO. However, DSC-MRI may provide additional insight into the MGMT methylation profile of primary IDHwild-type GBM. Thus, combined DKI and DSC-MRI provide promising potential biomarkers for glioma.Acknowledgements
No acknowledgement found.References
[1] Louis DN, Perry A, Reifenberger G, Deimling A von, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica 2016;131:803–20. doi:10.1007/s00401-016-1545-1[2] van Cauter S, Veraart J, Sijbers J, Peeters RR, Himmelreich U, Keyzer F de, et al. Gliomas: diffusion kurtosis MR imaging in grading. Radiology 2012;263:492–501. doi:10.1148/radiol.12110927
[3] Scott JN, Brasher PMA, Sevick RJ, Rewcastle NB, Forsyth PA. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 2002;59:947–9.
[4] Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 1992;34:463–9.
[5] Kulkarni AV, Guha A, Lozano A, Bernstein M. Incidence of silent hemorrhage and delayed deterioration after stereotactic brain biopsy. Journal of neurosurgery 1998;89:31–5. doi:10.3171/jns.1998.89.1.0031
[6] Reuss DE, Kratz A, Sahm F, Capper D, Schrimpf D, Koelsche C, et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta neuropathologica 2015;130:407–17. doi:10.1007/s00401-015-1454-8
[7] Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta neuropathologica 2015;129:867–73. doi:10.1007/s00401-015-1438-8
[8] Abedalthagafi M, Phillips JJ, Kim GE, Mueller S, Haas-Kogen DA, Marshall RE, et al. The alternative lengthening of telomere phenotype is significantly associated with loss of ATRX expression in high-grade pediatric and adult astrocytomas: a multi-institutional study of 214 astrocytomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2013;26:1425–32. doi:10.1038/modpathol.2013.90
[9] Pekmezci M, Rice T, Molinaro AM, Walsh KM, Decker PA, Hansen H, et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT // Adult infiltrating gliomas with WHO 2016 integrated diagnosis: Additional prognostic roles of ATRX and TERT. Acta neuropathologica 2017;133:1001–16. doi:10.1007/s00401-017-1690-1
[10] Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, Tribolet N de, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. The New England journal of medicine 2005;352:997–1003. doi:10.1056/NEJMoa043331
[11] Poot DHJ, den Dekker AJ, Achten E, Verhoye M, Sijbers J. Optimal experimental design for diffusion kurtosis imaging. IEEE transactions on medical imaging 2010;29:819–29. doi:10.1109/TMI.2009.2037915
[12] Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR in biomedicine 2010;23:698–710. doi:10.1002/nbm.1518
[15] Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS, Weisskoff RM, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 1994;191:41–51. doi:10.1148/radiology.191.1.8134596
[16] Sugahara T, Korogi Y, Kochi M, Ikushima I, Hirai T, Okuda T, et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR. American journal of roentgenology 1998;171:1479–86. doi:10.2214/ajr.171.6.9843274
Figures
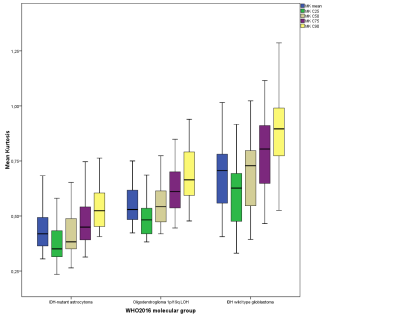
Box plots illustrate average, C25, C50, C75, and C90 histogram parameters of MK values in glioma grading according to their molecular profile based on 2016 CNS WHO.
MK is dimensionless.
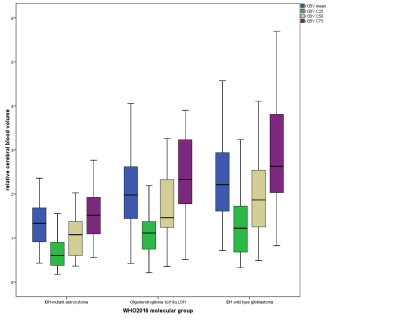
Box plots illustrate average, C25, C50, and C75 histogram parameters of rCBV values in glioma grading according to their molecular profile based on 2016 CNS WHO.
rCBV is
dimensionless.
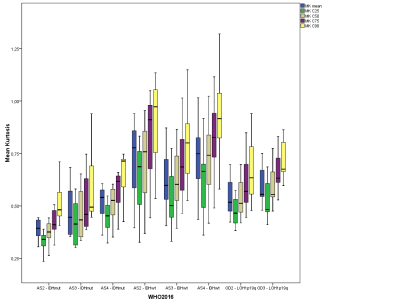
Box plots illustrate average, C25, C50, C75, and C90 histogram parameters of MK values in glioma grading according to 2016 CNS WHO-based individual tumor grades.
MK, mean kurtosis; MK is dimensionless
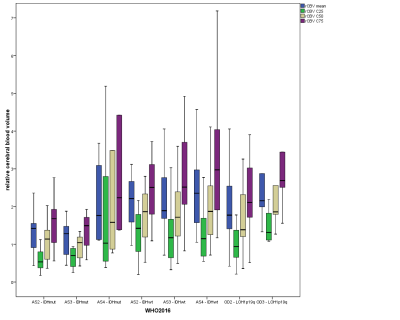
Box plots illustrate average, C25, C50, and C75 histogram parameters of rCBV values in glioma grading according to 2016 CNS WHO-based individual tumor grades.
rCBV is dimensionless