4846
Mutual Information: Depicting the Interdependence of Perfusion and Diffusion Magnetic Resonance Imaging in Glioblastoma Patients1Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom, 2Department of Neurosurgery, Shanghai General Hospital, Shanghai, China, 3Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 4Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom
Synopsis
The mismatch between energy demands of
Introduction
Glioblastoma represents the commonest malignant brain tumors, featured by profound heterogeneity and treatment resistance. A remarkably heterogeneous perfusion can be found in glioblastoma. The mismatch between energy demands of tumor growth and heterogeneous blood supply may cause variations in tumor microenvironment and is associated with tumor aggressiveness. A systematic method to study this global mismatch between cellularity and vascularity is crucial. Multi-parametric imaging may enable incorporation of complementary imaging modalities. However, due to the complex voxel distribution of the imaging modalities, finding validated surrogates to depict the interrelation between imaging modalities remains a challenge. Here we interrogate the inherent correlation between diffusion and perfusion magnetic resonance imaging (MRI) by using the mutual information (MI) of the two imaging modalities and evaluated its prognostic value in patient survival.Methods
Patients
We prospectively recruited 79 patients with supratentorial primary glioblastoma. Patients with history of previous brain tumor, cranial surgery, radiotherapy or chemotherapy were excluded. All subjects received maximal safe tumor resection and diagnosis was confirmed by pathology. After surgery, standard regimen of temozolomide chemoradiotherapy was performed. The Response Assessment in Neuro-oncology criteria was used to evaluate patients’ response. This study was approved by the local institutional review board.
Imaging processing and regions of interest
Multiparametric MRI sequences were pre-operatively acquired using a 3T MRI scanner. MRI sequences included T2-weighted, post-contrast T1-weighted and FLAIR images, as well as dynamic susceptibility contrast (DSC), diffusion weighted imaging (DWI), and multivoxel 2D 1H-MRS chemical shift imaging (CSI). All images were co-registered to T2W images. Apparent diffusion coefficient (ADC) maps were generated from DWI, whereas relative cerebral blood volume (rCBV) maps were generated from the DSC. Contrast-enhancing (CE) and non-enhancing (NE) regions of interest were manually delineated on the co-registered post-contrast T1-weighted and FLAIR images.
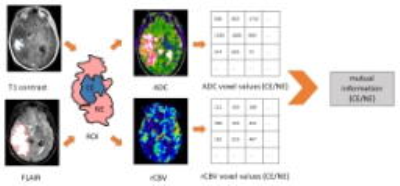
Mutual information between ADC and rCBV
The calculation of mutual information (MI) was demonstrated in Figure 1. ADC and rCBV values were extracted voxel-wise within the ROIs and thereafter treated as two random variables. To quantify the dependence of the two variables, MI (𝐴𝐷𝐶; rCB𝑉) was calculated as follows:
$$MI(ADC;rCBV) = \int_{rCVB}\int_{ADC} p(x,y)\log(\frac{p(x,y)}{p(x)p(y)})dxdy$$
where 𝑝(𝑥,𝑦) is the joint probability density function of ADC and rCBV, and 𝑝(𝑥) and 𝑝(𝑦) are the marginal probability density functions of ADC and rCBV. MI(𝐴𝐷𝐶;r𝐶𝐵𝑉) reflects the similarity between the joint distribution 𝑝(𝐴𝐷𝐶, 𝑟𝐶𝐵𝑉) and the factored distribution 𝑝(𝐴𝐷𝐶)𝑝(𝑟𝐶𝐵𝑉). An R package "infotheo" was used to perform the analysis.
Multivoxel MRS processing
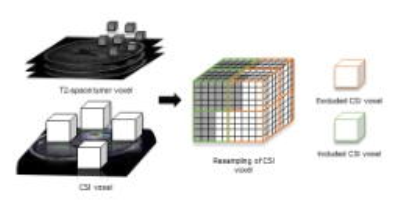
MRS data were processed using LCModel (Provencher, Oakville, Ontario) and the concentrations of metabolites were calculated as a ratio to creatine (Cr). The quality and reliability were evaluated using the values of Cramer–Rao lower bounds. Then T2-space pixels were projected to CSI space according to their coordinates. Only CSI voxels were included when the proportion of tumor pixels were over 75%. The weight of each CSI voxel was taken as the proportion of the tumor pixels in that CSI voxel. The summed weighted value was used as final metabolic value, as demonstrated in Figure 2.
Survival analysis
To evaluate the significance of MI (𝐴𝐷𝐶;r𝐶𝐵𝑉) to patient outcomes, we used Cox proportional hazards regression for survival analysis. All confounders, including IDH-1 mutation status, MGMT methylation status, sex, age, extent of resection and contrast-enhancing tumor volume, were considered.
Results
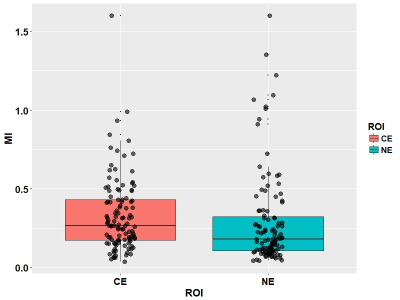
The median MI values were 0.27 (range 0.04-1.60) in the contrast-enhancing (CE) regions and 0.18 (range 0.04-1.60) in the non-enhancing (NE) regions, shown in Figure 3. The Pearson correlation showed significant positive correlation between these two measures (r=0.66, p < 0.001). For the MI in NE, Cox regression modelling showed that this variable significantly contributed to a worse overall survival (HR = 7.38, CI: 1.39- 39.2, p = 0.019). For the MI in CE, Cox regression modelling showed no significance. Pearson correlation test showed that the MI in the CE was significantly correlated the glutamine/creatine ratio (r=0.61, p = 0.015) and the N-acetylaspartate/creatine ratio (r=0.39, p < 0.001) in this region.Discussion
This study demonstrated that mutual information may describe the interrelation between the perfusion and diffusion imaging. The higher values of mutual information may contribute to a worse patient survival. Since ADC shows correlation with tumor cellularity in previous studies and rCBV is the established marker of vascularity, our current results suggested the high interdependence of the cellularity and vascularity may contribute to a more aggressive imaging phenotype. N-acetylaspartate is a maker of neurons and glutamine was reported to facilitate cancer cell migration. Our current CSI results suggested the selective stress caused by higher interdependence of cellularity and vascularity may increase the migration of tumor cells into the infiltrated tumor areas.Acknowledgements
No acknowledgement found.References
1. Reshef, D. N., et al. (2011). "Detecting Novel Associations in Large Data Sets." Science 334(6062): 1518.
2. Meyer, P. E. (2008). "Information-Theoretic Variable Selection and Network Inference from Microarray Data". PhD thesis of the Universite Libre de Bruxelles.
3. Cover, T. M. and Thomas, J. A. (1990). Elements of Information Theory. John Wiley, New York.
Figures