4845
Diagnostic Accuracy of 2-Hydroxyglutarate Magnetic Resonance Spectroscopy in Newly-Diagnosed Brain Mass and Suspected Recurrent Gliomas1Radiology, Brigham and Women's Hospital, Boston, MA, United States
Synopsis
Previous studies have reported the utility of 2-hydroxyglutarate magnetic resonance spectroscopy (2HG MRS) in diagnosing isocitrate dehydrogenase (IDH) status, which is of great value for patient management. We determined the optimal thresholds of single voxel spectroscopy (SVS) and chemical shift imaging (CSI) 2HG MRS in differentiating IDH-mutant gliomas from non-IDH-mutant controls, and then determined the diagnostic accuracy in two prospective cohorts of patients. We show that 2HG MRS provided diagnostic utility for IDH-mutant gliomas both preoperatively and at time of suspected tumor recurrence. Our findings may provide guidance for devising optimal MRS imaging protocol tailored to specific clinical settings.
Background
More than 80% of lower-grade gliomas (WHO grade II and III) and secondary glioblastomas harbor IDH mutations, leading to a more favorable prognosis1-3. Mutant IDHs result in accumulation of oncometabolite 2HG in IDH-mutated gliomas4. Recent advances in MRS technique have demonstrated that the signal of the oncometabolite 2HG can be measured in vivo using an echo time of 97 ms using both single voxel spectroscopy (SVS) and chemical shift imaging (CSI) methods5. However it is unclear what is the optimal threshold for 2HG detection and how it may be affected by these different methods in pre-operative and post-operative conditions. These are all important issues that would impact the clinical implementation of this methodology. Therefore, our goal is to optimize the threshold in a retrospective cohort of patients for each method in differentiating IDH-mutant gliomas from non-IDH-mutant controls, and then validate them in two prospective cohorts of 1) preoperative patients presenting with unknown brain mass, and 2) postoperative patients with known IDH-mutation status presenting with suspected recurrence to determine the diagnostic value of 2HG MRS under these two clinically relevant settings.Methods
All MRI and MRS exams were performed on one clinical 3.0 T MRI scanner (Siemens TIM Skyra, Erlangen, Germany) with a 32-channel head coil. SVS was acquired using PRESS TR/TE = 2000/97 ms, 128 averages, voxel size of 2x2x2 cm3. CSI was acquired using a semi-LASER sequence with TR/TE = 1700/97 ms, 160x160 cm2 field of view, 16x16 matrix, slice width of 15 cm, 3 averages, and weighted acquisition. The region of interest was localized on 3D FLAIR images to include as much of the lesion as possible while avoiding the surrounding tissue. Localized shimming was performed by adjustment of first- and second-order shim gradients using the automatic three-dimensional B0 field mapping technique followed by manual adjustment of the above-mentioned shim gradients to achieve a magnitude peak width of water at half-maximum of 14 Hz or less for SVS and 25Hz for CSI. All data was then processed using LCmodel (ver 6.2) using a custom basis that included 2HG and 20 metabolites. Signal-to-noise (SNR) ratio and full width at half maximum (FWHM) were used to assess the quality of the data. For CSI, the median and 75th percentile 2HG/Cr values of the selected voxels were then calculated for each subject. The post-processing was done without the knowledge of the patient IDH status which was determined using immunohistochemistry. The patient cohort consisted 1) discovery cohort (n=39) consisting of postoperative residual or recurrent glioma retrospectively identified from patients who had MRS, 2) prospective preoperative validation cohort (n=23), and 3) prospective recurrent-lesion validation cohort (n=15).Results
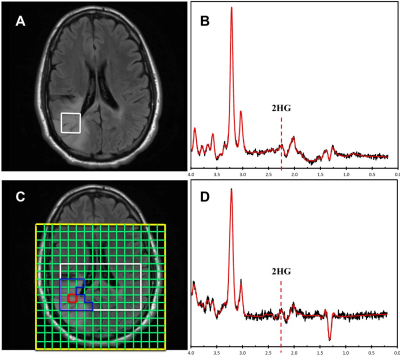
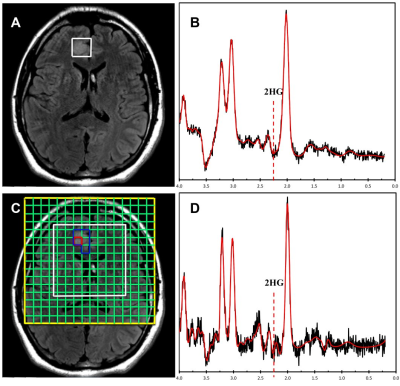
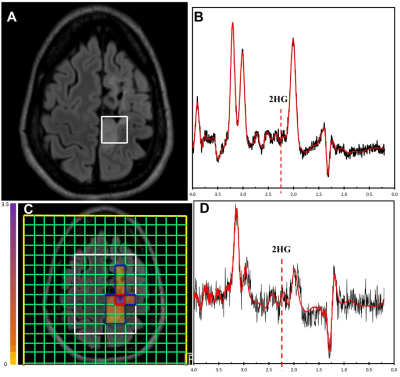
The optimal thresholds of 2HG/Cr in SVS and 75th percentile CSI for IDH mutations were 0.11 and 0.23, respectively (Figure 1). When applied to the validation sets, the sensitivity, specificity and accuracy in distinguishing IDH-mutant gliomas in the preoperative cohort were 83.33%, 100.00% and 93.33% for SVS, and 100.00%, 66.67% and 80.00% for CSI, respectively. In the recurrent-lesion cohort, the sensitivity, specificity and accuracy for discriminating IDH-positive recurrent gliomas were 50.00%, 42.86% and 45.45% for SVS, and 66.67%, 100.00% and 84.62% for CSI, respectively.Discussion
The threshold for SVS is similar to previous studies that reported a concentration of >1mM assuming that the ratio to creatine is approximately 8-10mM. The ratio of 2HG/Cr was used in order to accurately compare with CSI results which lacked a water reference due to its high cost in time and therefore normalized to creatine. In the pre-operative cohort, SVS performed quite well which is likely due to more homogeneous tumors that are not affected by treatment whereas CSI did not perform as well, likely due to the lower overall SNR as shown in Figure 2. However in the post-operative group, SVS methods did not perform as well whereas CSI appeared to perform better (Figure 3). Due to the larger spatial coverage and multi-voxel acquisition, the CSI has the advantage of accounting for the spatial heterogeneity of the abnormal tissues including gliosis, edema, and tumor which commonly co-exist in the post-treatment setting. Therefore utilization of the two methodologies appears to differ based on the clinical situation. It would appear the most efficient use would be to acquire both SVS and CSI in all cases.Conclusion
2HG MRS provides diagnostic utility for IDH-mutant gliomas both preoperatively and at time of suspected tumor recurrence. Diagnostic performance is dependent on treatment status where the ideal protocol would include both SVS and CSI for maximal diagnostic efficacy.Acknowledgements
This study was funded by the Brigham and Women’s Hospital Institute for the Neurosciences Seed Grant and the ARRS/ASNR Scholar Award. The authors would like to thank the China Scholarship Council for financial support.References
1. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. The New England journal of medicine. 2015; 372(26):2499-2508.
2. Cancer Genome Atlas Research N, Brat DJ, Verhaak RG, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. The New England journal of medicine. 2015; 372(26):2481-2498.
3. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. The New England journal of medicine. 2009; 360(8):765-773.
4. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009; 462(7274):739-744.
5. Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nature medicine. 2012; 18(4):624-629.
Figures