4840
Quantitative Cerebral Blood Flow Measured with Arterial Spin Labeling MRI in the Unaffected Contralateral Brain Hemisphere Predicts Outcome in Acute Ischemic Stroke1Stanford University, Stanford, CA, United States, 2Center for Stroke Research Berlin, Charité - Universitätsmedizin Berlin, Berlin, Germany, 3University of Pittsburgh Medical Center (UPMC), Pittsburgh, PA, United States, 4Swedish Medical Center, Seattle, WA, United States, 5Eden Medical Center, Castro Valley, CA, United States
Synopsis
During
Introduction
Arterial spin labeling (ASL) MRI can non-invasively measure quantitative Cerebral Blood Flow (CBF) and Arterial Transit Time (ATT).1 Evaluation of hemodynamics and penumbra in the ipsilateral hemisphere is a common practice for selecting patients for intra-arterial treatment (IAT).2,3 In this study, we hypothesized that the contralateral CBF (cCBF) may identify patients with high collateral capacity and better outcome.Methods
Patients were part of the prospective ‘iCAS’ (imaging the Collaterals in Acute Stroke) study.4 Inclusion criteria were (a) ischemic hemispheric stroke, (b) baseline NIHSS >= 1, (c) < 24 hours onset to imaging time [OIT], (d) age>=18, (e) informed consent, and (f) technically adequate imaging including DWI and 3D pseudocontinuous ASL with either a multi-delay acquisition [3T; 5 time points; PLDs 700-3000ms] or a single TI acquisition scheme [1.5T; PLD 1525 or 2025ms]. Outcomes were assessed by the National Institutes of Health Stroke Scale (NIHSS) at baseline, day 1, and day 5; and the modified Rankin Score (mRS) at day 30 and day 90. After image registration to the Montreal Neurological Institute (MNI) template, mean cCBF was calculated in Alberta stroke program early CT score (ASPECTS) ROIs in the contralateral hemisphere.
Patients were enrolled between November 2013 and August 2017, received a baseline MRI and were considered for endovascular intervention after imaging. General patient characteristics are reported as medians with interquartile ranges [IQR].
To assess the effect of cCBF for outcome prediction, patients were dichotomized by median cCBF into high (> 39 mL/100g/min) and low (<= 39 mL/100g/min) cCBF groups. We compared both groups statistically and controlled for the effects of clinical and imaging parameters: biological variables (age and gender), prior status (previous stroke event), acute imaging variables (onset to imaging time (OIT), initial baseline NIHSS, and acute DWI lesion size), and treatment type (tPA and IAT). Outcome differences were assessed with Wilcoxon (NIHSS) and Fisher’s exact test (mRS). Significance was reported with p<0.05.
A logistic regression model was applied to further investigate the relationship between cCBF and good neurological outcome at day 90 with mRS less than 3. We controlled for the effects of all clinical and imaging parameters which are available at the time of the initial baseline MRI (pre-therapy).
Results
Eighty patients met the inclusion criteria: 44 females, age 66 years [56-77], OIT 4.7 hours [3.4-7.6], baseline NIHSS 13 [9-20]. 43 underwent IAT [34 with final TICI >= 2b], mean cCBF 38.98 [31.6-44.5] mL/100g/min and cATT 1287 ms [1104-1455].
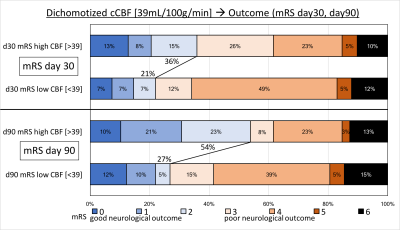
There was no difference between both groups in age, previous stroke, OIT, initial baseline NIHSS, acute DWI lesion size, tPA treatment, or IAT. Median NIHSS at baseline/day1/day5 for high and low cCBF groups was 13/8/5 and 13/14/11, respectively, which was significantly different on day 1 (p=0.027) and day 5 (p=0.010). Patients with higher cCBF were more likely to be female (p=0.047), have lower cATT (p=0.047) and better day 90 mRS (p=0.022) (see Figure 1 for detailed mRS distribution).
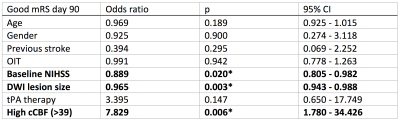
For logistic regression, results of each included variable - i.e., odds ratio, p-value, and confidence interval - are shown in Table 1 below. cCBF significantly predicts mRS day 90 outcome. Note that baseline NIHSS and DWI lesion size are two significant controlling factors (p<0.05): Patients with high cCBF presented with less severe baseline NIHSS and significantly smaller lesion size compared to those with low cCBF.
Discussion
Higher ASL cCBF at baseline MRI predicts better outcome in acute stroke independent of baseline NIHSS, DWI lesion size, and treatment type. This may reflect a better underlying capacity for collateral flow to the ischemic hemisphere. Quantitative ASL cCBF can be utilized as an objective prognostic imaging biomarker in acute stroke which does not have to rely on clinical examination. As a result of this study, patients with high cCBF might be eligible for intensified acute care following the initial baseline MRI.Conclusion
In acute stroke, higher CBF in the unaffected hemisphere is a predictive biomarker for better neurological outcome. Quantitative cCBF using ASL MRI should be investigated further to characterize its clinical and prognostic value.Acknowledgements
NIH R01-NS066506References
1 – Alsop D. C., Detre J. A., Golay X., et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015; 73,102–116.10.1002/mrm.25607.
2 – Borst J., Berkhemer O.A., Roos Y.B.W.E.M., et al. Value of computed tomographic perfusion-based patient selection for intra-arterial acute ischemic stroke treatment. Stroke 2015; 46(12),pp.3375-3382.
3 – Asadi H., Dowling R., Yan B., et al. Advances in endovascular treatment of acute ischaemic stroke. Intern Med J. 2015; 45(8):798-805. doi: 10.1111/imj.12652.
4 – Zaharchuk G., Marks M.P., Do H.M., et al. Introducing the Imaging the Collaterals in Acute Stroke (iCAS) Multicenter MRI Trial. Stroke 2015; 46:AWMP16.
Figures