4838
Correlation between Intracranial Artery Atherosclerotic Disease and the Integrity of Circle of Willis in Symptomatic Patients: A 3D MR Vessel Wall Imaging Study.1Department of Radiology, Beijing Tsinghua Changgung Hospital, Tsinghua University, Beijing, China, 2Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University School of Medicine, Beijing, China, 3Center for Brain Disorders Research, Capital Medical University and Beijing Institute for Brain Disorders, Beijing, China, 4Department of Radiology, University of Washington, Seattle, WA, United States
Synopsis
This study investigated the correlation between intracranial artery atherosclerotic disease and the integrity of communicating arteries in circle of Willis in symptomatic patients using MR imaging. We found that the intracranial artery stenosis was significantly associated with presence of anterior and posterior communicating arteries. Our findings suggest that the intracranial artery stenosis might be an independent indicator for the integrity of circle of Willis. Our data also suggest that, with the progression of intracranial artery stenosis, collateral circulation tends to be integrated from the anterior to posterior communicating arteries.
Introduction and Purpose:
In Chinese population, intracranial artery atherosclerotic disease is one of the major causes of ischemic stroke [1]. Circle of Willis (CoW) is considered to be the first level of collateral circulation which can compensate cerebral blood flow when cerebrovascular atherosclerotic stenosis is present. The integrity of CoW, representing the capability of compensation, was found to be associated with severity of ischemic stroke [2-4]. We hypothesized that intracranial artery atherosclerotic disease may stimulate the communicating arteries to integrate bilateral circulations with progression of intracranial atherosclerotic disease. This study sought to investigate the correlation between intracranial artery atherosclerotic disease and the integrity of communicating arteries in CoW in symptomatic patients using MR imaging.Methods
Study sample: Patients with recent cerebrovascular symptoms in anterior circulation were recruited. All the patients underwent MR vessel wall imaging for intracranial arteries. The study protocol was approved by institutional review board and written consent form was obtained from each patient. MR imaging: The MR imaging was performed on a 3.0T MR scanner (Achieva TX, Phillips Healthcare) with custom-designed 36-channel neurovascular coil. The MR imaging parameters were as follows: 3D MERGE: fast field echo (FFE), repeat time (TR)/echo time (TE) 9.2/4.3 ms, flip angle 6°, field of view (FOV) 4.0×16×25 cm3, and spatial resolution 0.8×0.8×0.8 mm3; 3D T1-VISTA: turbo spin echo, TR/TE 700/21 ms, FOV 4.5×20×20 cm3, and spatial resolution 0.6×0.6×0.6 mm3; 3D TOF: FFE, TR/TE 25/3.5 ms,flip angle 20°, FOV 4.5×20×20 cm3, and spatial resolution 0.7×0.7×1.4 mm3. Imaging review: Two experienced radiologists interpreted the MR images with consensus. Presence/absence, maximum wall thickness (Max WT), and stenosis of intracranial artery atherosclerotic plaque were determined. Luminal stenosis was measured on the maximum intensity projection (MIP) images of TOF MRA. Presence/absence of A1 segment of bilateral anterior cerebral arteries (ACA), anterior communicating artery (ACoA), P1 segment of bilateral posterior cerebral arteries (PCA) and bilateral posterior communicating arteries (PCoA) was evaluated. Statistical analysis: The Max WT and stenosis of intracranial plaques were compared between patients with and without ACoA or PCoA using non-parametric Mann-whitney test. The association between intracranial plaque features and presence of ACoA and PCoA was analyzed with logistic regression.Results
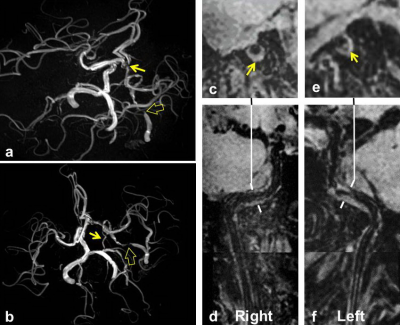
Of 110 patients (mean age: 57.2±11.1 years, 72 males), 51 (46.4%) and 44 (40%) had atherosclerotic plaques and stenosis in intracranial arteries, respectively. For integrity of CoW, we found that 100 (90.9%) had bilateral A1, 92 (83.6%) had bilateral P1, 91 (82.7%) had ACoA, and 58 (52.7%) had PCoA. For patients with both bilateral A1 and P1 (n=85), the intracranial stenosis in patients with ACoA was significantly greater than that of those without ACoA (19.7%±21.7% vs. 1.4%±3.3%, p=0.046). For patients with bilateral A1, P1 and ACoA (n=79), the intracranial stenosis in patients with PCoA was significantly greater than that of those without PCoA (27.9 %±23.7% vs. 13.5%±17.9%, p=0.007). Figure 1 represents an example for a patient with severe intracranial artery stenosis and bilateral A1, P1, ACoA, and PCoA. Logistic regression analysis revealed that the odds ratio (OR) of intracranial stenosis was 1.177 (95% CI, 1.051-1.317; p=0.005) with increment of 5% in predicting presence of PCoA in patients with bilateral A1, P1 and ACoA. After adjusted for confounding factors of gender, body mass index, hypertension, smoking, diabetes, and hyperlipidemia, this association remained statistically significant (OR, 1.282; 95% CI, 1.019-1.613; p=0.034). For 48 patients who had ACoA and intracranial plaques, the Max WT of intracranial plaques was similar in patients with and without PCoA (2.2±0.8mm vs. 2.2±0.7mm, p=0.748).Discussion and Conclusion
In the present study, intracranial artery stenosis was found to be significantly associated with presence of ACoA in patients with bilateral A1 and P1. In addition, we found that intracranial artery stenosis was significantly associated with presence of PCoA in patients with bilateral A1, P1 and ACoA. Our findings suggest that the intracranial artery stenosis might be an independent indicator for integrity of circle of Willis. Our data also suggest that, with the progression of intracranial artery stenosis, collateral circulation tends to be integrated from the anterior to posterior communicating arteries.Acknowledgements
None.References
1. Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45:663-669.
2. Routsonis KG, Stamboulis E, Christodoulaki M. Anomalies of the circle of Willis and atherosclerosis. Vasc Surg. 1973;7:141-145.
3. Hartkamp MJ, van Der Grond J, van Everdingen KJ, et al. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke. 1999;30:2671-2678.
4. Gutierrez J, Rosoklija G, Murray J, et al. A quantitative perspective to the study of brain arterial remodeling of donors with and without HIV in the Brain Arterial Remodeling Study (BARS). Front Physiol. 2014;5:56.
Figures