4835
Brain morphometric changes and functional connectivity alterations in post-stroke fatigue1Faculty of Health and Medicine, University of Newcastle, Newcastle, Australia, 2Department of Radiology, Lausanne University Hospital, Lausanne, Switzerland
Synopsis
Debilitating fatigue is the most common consequence of stroke, however there are no known clinical or radiological biomarkers associated with post-stroke fatigue. We assessed differences in regional brain volumes obtained from T1-weighted, high-resolution structural scans between stroke survivors with and without severe fatigue. Differences were observed in the volume of the globus pallidus and putamen, as well as the ipsilesional temporal, parietal and frontal lobe. The mentioned morphological differences between stroke survivors with and without severe fatigue have also been reported in multiple sclerosis and Parkinson’s-related fatigue, suggesting a possible common mechanism.
Introduction
Debilitating fatigue is one of the most common consequences of stroke and is experienced by approximately 40% of stroke survivors1. Previous studies have shown that there is no link between baseline stroke severity, stroke location, or the resulting disability at predicting the onset of fatigue. In the present study, we aimed at using comprehensive radiological assessments to identify if there were differences between fatigued and non-fatigued stroke survivors in terms of morphometry or functional connectivity.Methods
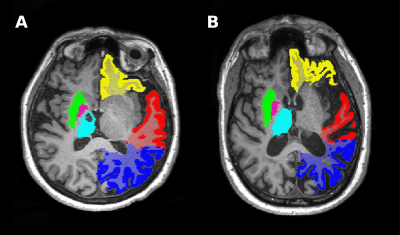
Stroke survivors were recruited from stroke clinics by neurologists at the John Hunter Hospital, NSW, Australia. Included participants had a history of ischemic or hemorrhagic stroke more than 3 months prior to inclusion. Self-reported fatigue was assessed using the multidimensional fatigue (MFI) scale. Severe fatigue was defined as an MFI ≥ 60, and low fatigue as ≤ 50. Participants underwent a multi-modal scanning session in which a structural multi-echo MPRAGE (MEMPRAGE) with a 1mm-isotropic resolution and a functional resting-state EPI (3mm isotropic, 250 volumes over 10 minutes) at 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). Images of participants with left-hemispheric strokes were flipped, such that data could be presented in terms of ipsilesional and contralesional values. MEMPRAGE data were processed using the fully automated MorphoBox prototype2. Resulting brain volumes for low- and severe-fatigue groups were compared using independent t-tests with bootstrapping or the Mann-Whitney U test (using a significance level of 0.05) with results presented with and without correction for total intracranial volume (cTIV). Regions-of-interest (ROIs) for a seed-based inter-hemispheric functional connectivity assessment were chosen from regions that were found to be significantly different from the severely fatigued and low fatigued volumetric analysis. Resting-state data were analyzed in the Nipype framework and were despiked (AFNI), slice-time corrected (SPM), motion corrected using the 24-parameter model3 and registered to MNI-space (ANTs). Subsequently, data were band-pass filtered (0.01 – 0.08 Hz) and smoothed with a Gaussian kernel with a 6mm FWHM (SPM). To calculate inter-hemispheric functional connectivity for seed-based analysis, sub-cortical ROIs were obtained using segmentation by FSL's FIRST. Cortical ROIs were created in FSLeyes from probability maps provided in the Oxford-Harvard brain atlas. Inter-hemispheric functional connectivity was estimated as the Fisher-Z transformed correlation coefficient between ipsilesional and contralesional ROI time-series.Results
Structural data from 45 participants and resting-state data from 40 participants were included in the study. There were 31 patients with severe fatigue (MFI: 72.3 ± 8.90; mean age: 63.7 ± 7.56) and 14 without fatigue (MFI: 37.2 ± 9.21; mean age: 63.6 ± 13.9). Fatigued patients had significantly smaller ipsilesional caudate nucleus (mean difference + SE = -0.934 ± 0.320ml; p=0.018, corrected for total intracranial volume, cTIV p=0.097), hippocampus (-0.348 ± 0.130ml; p=0.009, cTIV p=0.249), amygdala (-0.250 ± 0.102ml; p=0.030, cTIV p=0.027), and thalamus (-0.980 ± 0.349ml; p=0.010, cTIV p=0.083) volumes. The putamen was significantly smaller in both the ipsilesional and contralesional hemisphere (-1.698 ± 0.599ml; p=0.003, cTIV p=0.023) as well as the globus pallidus (-0.311 ± 0.122ml; p=0.028, cTIV p=0.031). Cortical white matter volumes were significantly reduced in patients with severe fatigue for the ipsilesional frontal (-20.389 ± 7.291ml; p=0.010, cTIV p=0.028), temporal (-14.050 ± 4.662ml; p=0.008, cTIV p=0.012), and parietal (-13.899 ± 4.631ml; p=0.009, cTIV p=0.006) cortices. Inter-hemispheric functional connectivity was significantly lower in the severe-fatigue group in the orbitofrontal cortex (-0.242 ± 0.095; p = 0.012). No further significant differences in inter-hemispheric functional connectivity were observed in any of the other cortical and sub-cortical ROIs.Discussion
Stroke survivors with high levels of self-reported fatigue have reduced volumes of structures related to the fronto-striatal network, which include the ipsilesional basal ganglia and frontal, temporal and parietal cortices. Concurrent differences in inter-hemispheric functional connectivity were also observed in the orbitofrontal cortex. The volume differences between fatigued and non-fatigued patients in this study were found mostly in the basal ganglia and deep white mater remote to the initial infarct, suggesting that fatigue may result from a global phenomenon rather than the localized effects of infarction. The basal ganglia and fronto-striatal circuit involvement have previously been implicated in fatigue for other diseases such as multiple sclerosis4 and Parkinson's disease5, suggesting that fatigue may have a similar pathophysiology across other central nervous diseases.Conclusion
These results suggest that post-stroke fatigue may be associated with global volumetric changes after stroke around the fronto-striatal network. Previous studies, which only included clinical variables and basic demographic information, have failed to demonstrate such associations with fatigue, supporting need for comprehensive imaging-based assessments.Acknowledgements
References
1. Hinkle J, Becker K, Kim J, et al. Post-stroke fatigue: emerging evidence and approaches to management: A Scientific Statement for Healthcare Professionals From the American Heart Association. Stroke. 2017;48(11):e000-e000
2. Schmitter D, Roche A, Maréchal B, et al. An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. Neuroimage Clin. 2015;7:7–17
3. Friston KJ, Williams S, Howard R, et al. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35(3):346-55.
4. Finke C, Schlichtling J, Papazoglou, et al. Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult Scler J. 2015;21(7):925-34
5. Pavese N, Metta V, Bose S, et al. Fatigue in Parkinson's disease is linked to striatal and limbic serotonergic dysfunction. Brain. 2010;133(11):3434-43
Figures