4834
Carotid stenosis: a risk factor for white matter disease even at presymptomatic stage1Department of Physics, FFCLRP, University of São Paulo, Ribeirão Preto, Brazil, 2Department of Neurosciences and Behavioral Science, FMRP, University of São Paulo, Ribeirão Preto, Brazil, 3Department of Computing and Mathematics, FFCLRP, University of São Paulo, Ribeirão Preto, Brazil
Synopsis
Studies have suggested that cerebral white matter hyperintensity (WMH) is due to hypertension and is associated with carotid artery stenosis (CAS). However, it is unclear whether this association is attributable to effects on WM and how asymptomatic CAS contributes to it. Therefore, we aimed to assess the association between ACAS and WMH lesions and its relationship with cognitive decline using MRI to provide information that may help predicting cases at risk of brain ischemia. Our data showed that ACAS is associated with WMH lesions and cognitive decline, indicating that ACAS, in addition to age, is likely to cause WM lesions.
Introduction and Purpose
Cerebral white matter hyperintensity (WMH) lesions are common with advancing age and are associated with cognitive decline in elderly individuals. Longitudinal studies have demonstrated that WMH increases the risk of incident stroke, dementia, and mortality1. Particularly, WMH is a usual finding on fluid attenuation inversion recovery (FLAIR-MRI) images of elderly subjects. Although the pathogenesis of WMH is poorly understood and is probably multifactorial, studies have suggested that WMH is largely due to hypertension and is associated with carotid artery stenosis (CAS), considered one of the major causes of ischemic stroke and associated with cognitive impairment1. However, it is unclear whether this association is attributable to effects on WM2 and how asymptomatic CAS contributes to it. Therefore, the present study aimed to assess the association between ACAS and WMH lesions and its relation with cognitive decline using MRI to provide information that may help predicting cases at risk of brain ischemia.Material and Methods
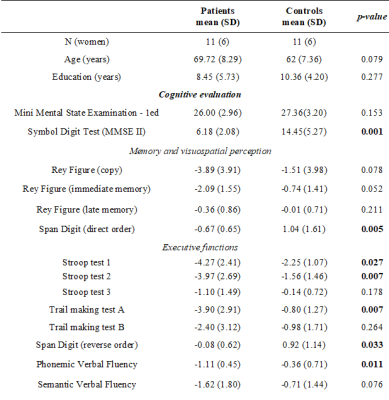
Eleven elderly patients with severe (≥70%) unilateral ACAS were compared with 11 healthy elderly controls using a comprehensive neuropsychological battery and FLAIR images. All subjects participated in this study after reading and signing an informed consent approved by the Ethics in Research Committee of the Clinical Hospital of Ribeirao Preto. Cognitive assessment included Mini Mental State Examination (MMSE I), Symbol Digit Test (MMSE II), Digit Span, Trail Making Test A and B, Stroop Test, Phonemic and Semantic Verbal Fluency Tests and Complex Rey Figure (copy, immediate and late memory). MRI was acquired on a 3T system (Philips) using 32-channel head coil for reception. For anatomical reference, images were acquired using a 3DT1W GRE sequence (TR/TE=7/3.1 ms, FA=8°, FOV=240x240 mm2, 160 1-mm slices). FLAIR images were collected using a 2D fast SE sequence (TR/TE=8000/120 ms, TI=2 s, matrix=256x256, FOV=240x240 mm2, 42 3-mm slices, no gap), and was preprocessed to remove brain volume (FSL-BET)3, attenuate noise level using AAD filter4, correct bias field inhomogeneities (N4 filter)5 and normalize to a T1 space using rigid registration strategy. Then, WMH lesion load (WMH-LL) estimate was performed in SPM12 applying an automatic algorithm based on parametric signal comparison with atlas (LST-LPA)6. Each lesion mask was visually inspected to correct for minor lesion segmentation artifacts. Lesion load was obtained for regions of WM tracts (WMT). Cognitive scores and WMH-LL comparison between groups was performed with Mann-Whitney U Test. Correlations between the scores and WMH-LL were performed using the Spearman correlation coefficient. Statistical analysis was performed in STATA14 Software7.Results
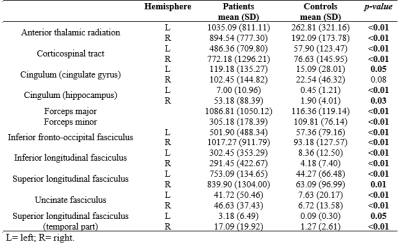
Characteristics of the participants are summarized in Table 1. Compared with healthy controls, patients had worse information processing speed scores, poorer memory and complex visuospatial performances and impaired executive functions. Moreover, patients had higher WMH-LL (p<0.05) in almost all studied tracts (Table 2 and Figure 1). When comparing hemispheres ipsi and contralateral to the stenosis (patient group), WMH-LL in the cingulum (hippocampus) tract showed significant difference between hemispheres (ipsi: 52.27(88.90); contra: 7.90(11.03); p=0.036). In the control group, WMH-LL correlated with global cognition, memory, visuospatial and executive function measures (p<0.01), while in the patient group the correlations reduced in number of tracts and cognitive tests involved, with lower correlations with global cognition and executive function measures (p<0.05) (Table 4). Interestingly, age was associated with WMH-LL only for the control group.Discussion
Subjects with ACAS showed substantial deficits on tasks of mental speed, memory, visuospatial abilities and executive functions. Compared with healthy controls, patients had higher WMH-LL in almost all studied tracts. Even the controls presented WMH lesions, as showed in previous studies of aging2. However, the presence of ACAS significantly increased the lesion load, indicating that it is associated with WMH, which can lead to cognitive decline. Additionally, difference in WMH-LL estimates was found between the hemispheres ipsilateral and contralateral to the stenosis in the cingulum tract in patients. The cingulum takes memory information and integrates it to other parts of the brain. Damage to the cingulum also damages the hippocampus, which is pivotal in memory storage8. In general, we found that WMH were associated with increased decline in global cognition, perceptual speed, memory and executive functions when comparing patients with controls. These findings suggest that WM damage contribute to decline in multiple cognitive systems in elderly. Moreover, it is interesting to note that age correlated with WMH-LL only in controls, indicating that CAS even at presymptomatic stage is more important than age in the contribution to WMH-LL.Conclusion
Our data showed that ACAS is associated with WMH lesions and cognitive decline, indicating that ACAS, in addition to age, is likely to cause white matter lesions.Acknowledgements
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES, Brasil.References
1. Kandiah, N., Goh, O., Mak, E., Marmin, M. & Ng, A. Carotid stenosis: A risk factor for cerebral white-matter disease. J. Stroke Cerebrovasc. Dis. 23, 136–139 (2014).
2. Ammirati, E. et al. Relation between characteristics of carotid atherosclerotic plaques and brain white matter hyperintensities in asymptomatic patients. Sci. Rep. 7, (2017).
3. Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155.
4. Senra Filho, A. C., Garrido Salmon, C. E., & Murta Junior, L. O. (2015). Anomalous diffusion process applied to magnetic resonance image enhancement. Physics in Medicine and Biology, 60(6), 2355–2373.
5. Sled, J. G., Zijdenbos, A. P., & Evans, A. C. (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging, 17(1), 87–97.
6. Schmidt, P., Gaser, C., Arsic, M., Buck, D., Förschler, A., Berthele, A. & Mühlau, M. (2012). An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. NeuroImage, 59(4), 3774–83.
7. StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.
8. Metzler-Baddeley, C; Jones, DK; Steventon, J; Westacott, L; Aggleton, JP; O'Sullivan, MJ (December 2012). "Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment". J Neurosci. 32 (49): 17612–9.
Figures