4832
Cerebrovascular resistance responses to CO2 improve after revascularization surgery1Division of Neuroradiology, Joint Department of Medical Imaging, University Health Network, Toronto, ON, Canada, 2Institute of Medical Science, University of Toronto, Toronto, ON, Canada, 3The Russell H. Morgan Department of Radiology & Radiological Science, The John Hopkins University School of Medicine, Baltimore, MD, United States, 4Department of Anaesthesia and Pain Management, University Health Network, Toronto, ON, Canada, 5Department of Physiology, University of Toronto, Toronto, ON, Canada
Synopsis
The cerebral hemodynamics of patients undergoing revascularization surgery for intracranial steno-occlusive disease (IC-SOD) were assessed by deriving an estimate of their cerebrovascular resistance response to CO2 from their BOLD response to CO2. Significant improvements were found in the sigmoid parameters describing their resistance responses.
Purpose:
To assess changes in cerebrovascular resistance responses to CO2 in patients before and after revascularization surgery. Anatomical maps of resistance response sigmoid parameters were calculated from BOLD responses to a CO2 ramp stimulus (hypocapnia to hypercapnia) using a model of vascular interactions. This novel measurement of cerebrovascular hemodynamics based on resistance may provide an improved objective metric to assess the efficacy of revascularization surgery.Introduction:
Cerebrovascular reactivity (CVR) is the change in cerebral blood flow (CBF) in response to increases in the arterial partial pressure of CO2 (PaCO2), and is used to assess cerebral hemodynamic impairment. In a healthy brain, the association between CBF and PaCO2 is sigmoidal in shape, whereas patients with intracranial steno-occlusive disease (IC-SOD) typically show regions where CBF decreases in response to CO2. This paradoxical response is known as the vascular steal phenomenon, where blood flow is redistributed from regions of exhausted vasodilatory reserve to those with intact vasodilatory reserve able to decrease their resistance1.
Previous studies show that surgical revascularization can reverse cortical thinning in areas of steal 2, improve CVR in ipsilateral and contralateral hemispheres 3-4, and improve cognitive function.5-7 However, CVR measures only the linear response slope, and so we sought details of how the vascular resistance response is affected after revascularization surgery. To answer this question, we used a model of the interactions between two parallel vascular beds whose resistances changed sigmoidally with CO2. Unlike flow responses, resistance responses are unconfounded by the cerebrovascular network flow interactions that influence the shape of the BOLD-CO2 response and CVR.
Methods:
We used a ramp CO2 stimulus to survey the full extent of blood flow responses including nonlinearities. 11 patients were recruited (2 were removed due to unusable data). Of this total, 6 had a unilateral extracranial-intracranial (EC-IC) bypass, 1 a bilateral EC-IC bypass, and 1 patient an encephaloduroarteriosynangiosis (EDAS) revascularization surgery). All were scanned on a 3-Tesla GE system MRI scanner using an eight-channel phased array head coil at Toronto Western Hospital. The ramp CO2 stimulus sequence was programmed into a computer controlled gas blender (RespirActTM) that ran a prospective gas-targeting algorithm 8, which controlled the CO2 stimulus such that PETCO2 was equivalent to PaCO2.9-10
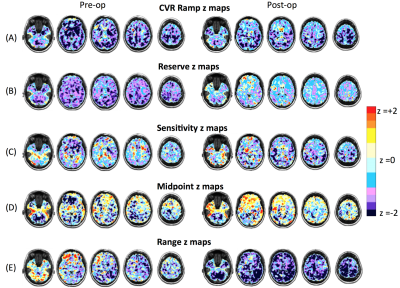
Statistical z-map analysis on voxels 2 standard deviations (SD) below the reference atlases for resistance sigmoid parameters and ramp CVR in both gray matter (GM) and white matter (WM) were assessed for pre- and post-conditions.
Results:
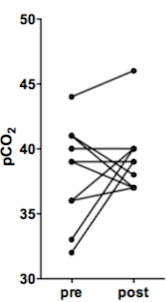
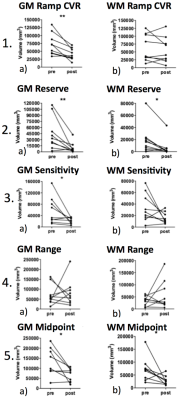
A paired-t-test revealed no significant changes in resting PETCO2 values after surgery, p = 0.44, although a trend towards 39 mmHg was observed (Figure 1). Ramp CVR improved in GM after surgery (p < 0.001) (Figure 2.1a), but not in WM (Figure 2.1b). Resting resistance reserve, the decrease in resistance from resting PETCO2 to full vasodilation improved in GM (p<0.001) (Figure 2.2a) and in WM (p<0.05) (Figure 2.2b). Resting resistance sensitivity, the slope of the resistance sigmoid CO2 response at resting PETCO2, improved in GM (p<0.05) (Figure 2.3a), but not in WM (Figure 2.3b). There was no change in resistance sigmoid range in both GM and WM (Figure 2.4a/b) after surgery. Resistance sigmoid midpoints, the PETCO2 at the sigmoid inflexion point where slope (sensitivity) is maximum, showed improvement, changing towards the reference resistance midpoint (39 mm Hg) in both GM and WM after surgery (p<0.05) (Figure 2.5a/b).Discussion and Conclusion:
This study is the first to compare the application of a novel measure of cerebral hemodynamics based on sigmoidal vascular resistance profiles. The advantage of the resistance model is that it is not influenced by non-linear responses commonly caused by the interaction of vascular beds during a global CO2 stimulus. This is evident in the improvement in white matter resistance post surgery compared to the conventional flow based CVR analysis as seen in Figure 1b. and 2b. We found significant improvements in resting resistance reserve for GM and WM, a metric that may be the best identifier of pathophysiology since it shows regions that have exhausted their vasodilatory reserve and are unable to respond to vasodilatory demands.
Resting resistance sensitivity significantly improved in GM after surgery, indicating an increase in the vasculature’s ability to regulate CBF in response to vasodilatory demands. Resistance sigmoid midpoints significantly improved after surgery in both GM and WM; changing towards the midpoint PetCO2 of the healthy reference sigmoid midpoint. Differences in midpoint from the control atlas for the pre-op maps may indicate an adaptation of resistance regulation to accommodate chronic underperfusion and acidosis (higher midpoint) or chronic over-perfusion and alkalosis (lower midpoint). In summary, our findings show that revascularization surgery in IC-SOD patients results in significant improvements in cerebral resistance hemodynamics on the first post-op assessment.
Acknowledgements
We acknowledge Abby Skanda with her assistance in patient recruitment and Eugen Hlasny with his assistance in MRI scanning.References
1. Sobczyk O, Battisti-Charbonney A, Fierstra J, Mandell DM, et al. A conceptual model for CO2-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. Neuroimage. 2014;92: 56–68.
2. Fierstra J, Maclean DB, Fisher JA, et al. Surgical Revascularization Reverses Cerebral Cortical Thinning in Patients With Severe Cerebrovascular Steno-Occlusive Disease. 2011;42(6):1631-1637. doi:10.1161/STROKEAHA.110.608521.
3. Sam K, Poublanc J, Sobczyk O, et al. Assessing the effect of unilateral cerebral revascularisation on the vascular reactivity of the non-intervened hemisphere: a retrospective observational study. BMJ Open. 2015;5:e006014.
4. Mandell DM, Han JS, Poublanc J, et al. Quantitative Measurement of Cerebrovascular Reactivity by Blood Oxygen Level-Dependent MR Imaging in Patients with Intracranial Stenosis: Preoperative Cerebrovascular Reactivity Predicts the Effect of Extracranial-Intracranial Bypass Surgery. Am J Neuroradiol. 2011;32(4):721–727.
5. Baek HJ, Chung SY, Park MS, et al. Preliminary study of neurocognitive dysfunction in adult moyamoya disease and improvement after superficial temporal artery-middle cerebral artery bypass. J Korean Neurosurg Soc. 2014;56(3):188–193.
6. Bowen M, Marks MP, and Steinberg GK. Neuropsychological recovery from childhood moyamoya disease. Brain Dev. 1998;20(2):119–123.
7. Sasoh M, Ogasawara K, Kuroda K, et al. Effects of EC-IC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surg Neurol. 2003;59(6)455-460.
8. Slessarev M, Han J, Mardimae A, et al. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J Physiol. 2007; 58(Pt 3):1207–1219.
9. Ito S, Mardimae A, Han J, Duffin et al. Non-invasive prospective targeting of arterial P CO 2 in subjects at rest. J Physiol. 2008;586(15):3675–3682.
10. Willie CK, Macleod DB, Shaw AD, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol. 2012;590(14):3261–3275.
Figures