4824
Multi-parametric MR Microscopy of Cerebral Thrombi as a Tool for Prediction of Thrombectomy Procedure Times in Stroke Therapy1Jožef Stefan Institute, Ljubljana, Slovenia, 2Universitiy of Ljubljana, Institute of Physiology, Ljubljana, Slovenia, 3Universitiy of Ljubljana, Institute of Forensic Medicine, Ljubljana, Slovenia, 4University of Ljubljana, Institute of Patophysiology, Ljubljana, Slovenia, 5University Medical Center Ljubljana, Clinical Institute of Radiology, Ljubljana, Slovenia, 6General hospital Slovenj Gradec, Department for interventional and diagnostic radiology, Slovenj Gradec, Slovenia
Synopsis
In this study human cerebral thrombi were quantitatively characterized after their acquisition by mechanical thromectomy. The characterization was based on multi-parametric MRI using 3D T1-weighted imaging and ADC and T2 mapping. In the study it was shown that thrombi complex structure can be assessed by ADC and T2 mapping MRI mapping techniques and that the MRI maps of thrombi can be used for prognosis of the mechanical thrombectomy procedure times prior to the interventions.
Introduction
Acute cerebral
artery occlusions are becoming the leading cause for a stroke [1]. Treatment of the acute stroke is usually based on various recanalization
approaches, namely, administration of thrombolytic agents [2] or mechanical
removal of thrombi known as thrombectomy [3]. Various origins of cerebral
thrombi may in general lead to the formation of highly diverse thrombi, which
may have consequently different mechanical properties and also differ in susceptibility
to thrombolysis.
Microscopically, thrombi are composed of platelets and red blood
cells (RBCs) interspersed within the fibrin meshwork. Fibrin meshwork by itself
is a permeable porous structure. However, its permeability is strongly
influenced by the presence of platelets as well as of fibrin cross-linking [4]. Entrapped RBCs in the thrombi reduce pore sizes within the meshwork and therefore
also reduce the permeability of thrombi. An accurate assessment of thrombi structure and
composition is of a high importance in treatment planning and prognosis of
recanalization [5]. Thrombi can well be characterized by MRI, specifically by the apparent diffusion coefficient (ADC) and by the transverse relaxation time (T2) mapping techniques. The techniques are sensitive to differences
in water mobility and NMR relaxation parameters between RBC-rich and
platelet-rich regions and were already proven in assessment of the thrombolytic outcome of venous thrombi [6]. This study aims to
quantitatively characterize human cerebral thrombi by multi-parametric MR microscopy and to correlate these results with the corresponding mechanical thrombectomy
procedure times in which the thrombi were acquired.
Materials and Methods
Cerebral thrombi for the study (n = 28) were acquired by mechanical thrombectomy from patients diagnosed with acute stroke due to occluded middle
cerebral arteries. All samples were rinsed with isotonic saline of 0.9% w/v
of NaCl, pH 7.4 and closed in Teflon tubes to prevent tissue desiccation during
MR scanning. For all samples, MRI protocol was performed within 24 hours after
the thrombectomy.
MR imaging
was performed on a 100 MHz MRI scanner (Tecmag, Houston TX) equipped with a micro-imaging accessories (Bruker,
Ettlingen, Germany) for MR microscopy. The samples were analyzed by a multi-parametric MRI
protocol consisting of the 3D T1-weighted MRI using spin-echo imaging sequence, followed by the DWI
sequence for ADC mapping and the multi-spin-echo imaging
sequence for T2
mapping. The samples were scanned with filed of view of 20 x 10 x 10 mm3 and the imaging matrix of 128 x 64 x 16 that yield in plane imaging resolution of 150 μm and slice thickness of 600 μm. ADC maps were calculated from DW images with b values of
0,
260, 620, 1250 mm2/s and T2 maps were calculated from images in eight consecutive echoes with the inter echo time of 16 ms. For the samples average ADC and T2 values were calculated and then correlated to the corresponding thrombectomy procedure times.
Results and Discussion
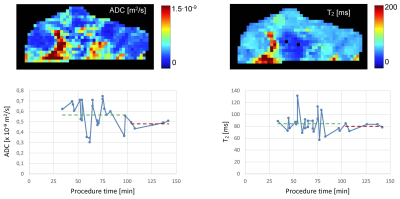
As can be seen from the ADC and T2 maps of a representative cerebral thrombus in Figure 1 (top row), the thrombi are compositionally heterogeneous and can be characterized by different regions with respect to water mobility that correspond to different ranges of ADC and T2 values. Figure 1 (bottom row) depicts dependence of sample average ADC and T2 values on the thrombectomy procedure times. From both graphs it can be seen that with longer procedure times (PT) both ADC and T2 values decrease. Results of linear regression analysis are: ADC = -0,0014 x 10-9 mm2/s/min PT + 0,66 x 10-9 mm2/s and T2 = -0.103 ms/min PT + 91 ms. The analysis also showed that average ADC values for procedure times less than 100 min are statistically significantly different from the ADC values for procedure times more than 100 min (0.57 ± 0.13 x 10-9 mm2/s vs. 0.48 ± 0.03 x 10-9 mm2/s; p = 0.009). The discrimination between the groups of thrombi is not as efficient with T2 values were the differences were not statistically significant (84 ± 17 ms vs. 80 ± 5 ms; p = 0.32).Conclusion
The study confirmed that ADC and T2 mapping are two MRI methods capable of distinguishing among cerebral thrombi of different complexity and composition. It was already known that these two properties along with the size of the thrombi critically determine their susceptibility to thrombolysis. However in this study we have shown that these properties to a large extent determine also the procedure time of mechanical thrombectomy and can be therefore used for its prediction. The prediction for the procedure time can be further improved by using a predictive models combining all three relevant parameters: ADC and T2 values along with the thrombus size.Acknowledgements
This study was financially supported by the Slovenian Research Agency (ARRS) project J3-7518.References
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: Heart disease and stroke statistics-2012 update a report from the american heart association. Circulation. 2012;125:188-197 2.
- Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. New Engl J Med. 2015;372:11-20 3.
- Xavier AR, Farkas J. Catheter-based recanalization techniques for acute ischemic stroke. Neuroimag Clin N Am. 2005;15:441.
- Bajd F, Vidmar J, Fabjan A, Blinc A, Kralj E, Bizjak N, et al. Impact of altered venous hemodynamic conditions on the formation of platelet layers in thromboemboli. Thromb Res. 2012;129:158-16
- Yuki I, Kan I, Vinters HV, Kim RH, Golshan A, Vinuela FA, et al. The impact of thromboemboli histology on the performance of a mechanical thrombectomy device. Am J Neuroradiol. 2012;33:643-648.
- Vidmar J, Blinc A, Sersa I. A comparison of the adc and t-2 mapping in an assessment of blood-clot lysability. Nmr Biomed. 2010;23:34-40.
Figures