4821
Prognostication of stroke recovery using structural connectivity1Department of Diagnostic Radiology, The University of Hong Kong, HKSAR, China, 2Department of Medicine, The University of Hong Kong, HKSAR, China, 3Department of Rehabilitation Medicine, Tung Wah Hospital, HKSAR, China, 4Department of Occupational Therapy, Tung Wah Hospital, HKSAR, China, 5The State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, HKSAR, China, 6Philips Healthcare, HKSAR, China
Synopsis
We aim to investigate the longitudinal changes in the structural brain network of patients with acute subcortical ischemic infarct in the motor system, and the relation between motor recovery and network measures. Our results showed that the nodal degree of parahippocampus, amygdala, calcarine fissure, cuneus and fusiform gyrus increased with time after stroke, and that network topology measured at acute phase was associated with the recovery of motor function at 6 months after stroke. These findings suggested that network topology could potentially be a prognostic indicator of motor recovery for patients with acute subcortical ischemic infarct in the motor system.
Purpose
There is a desperate need to further our understanding of the mechanisms underlying the recovery of brain function after stroke in a hope to inform improved rehabilitative approaches. While reorganization in functional brain network after rehabilitation was previously demonstrated 1,2, it is uncertain whether that in the structural brain network occurs. We therefore aim to investigate the longitudinal changes in the structural brain network of patients with acute subcortical ischemic infarct in the motor system, and investigate the relation between motor recovery and network measures.Materials and Methods
Participant Patients with acute subcortical ischemic infarct in the motor system were recruited (n = 15). Those with history of neuropsychological disorders and cognitive impairments were excluded. All patients received routine rehabilitation at about a week after stroke onset.
Experiment Diffusion MRI data were acquired at less than a week, and 1, 3 and 6 months after stroke onset. DWIs were acquired using single-shot EPI with b-value of 1000 s/mm2 along 32 directions, and 3.0T Achieva TX (Philips Healthcare). Barthel Index and Upper-Extremity Fugl-Meyer scale (UE-FM) were obtained on the same day as MRI for functional and motor assessments, respectively.
Data analysis MPRAGE images were segmented into 90 brain regions according to the AAL atlas. Whole-brain tractography and network measures were obtained using Diffusion Toolkit 3 and Brain Connectivity Toolbox 4, respectively.
Statistical analysis Repeated measures one-way ANOVA with Bonferroni post-hoc analysis was performed to investigate the longitudinal changes in structural brain network. Associations between functional and motor assessments versus network measures were determined using Spearman correlation. All analyses were controlled for age, sex, total small vessel disease (SVD) score (to account for the burden of SVD lesions) 5 and the betweenness centrality of the brain region of age-matched healthy control that colocalized with infarct (to account for the variability in the load and location of infarct).
Results
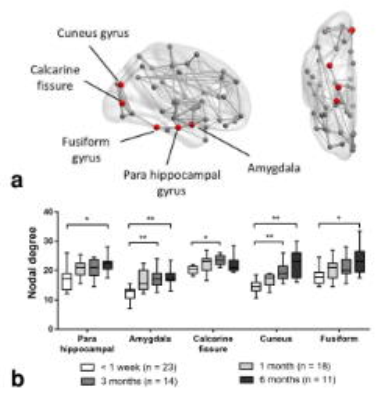
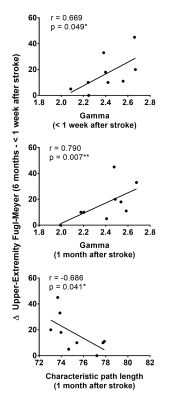
Compared to the structural brain network measured at less than a week after stroke (Figure 1), the nodal degree of amygdala (p = 0.004), calcarine fissure (p = 0.041) and cuneus (p = 0.006) increased at 3 months after stroke, and also that of parahippocampal (p = 0.007), amygdala (p = 0.017), cuneus (p < 0.001) and fusiform gyrus (p = 0.035) at 6 months. As shown in Figure 2, the change between the UE-FM at 6 months and that at less than a week was associated with the normalized clustering coefficient (γ) at less than a week (r = 0.669, p = 0.049), and the γ (r = 0.790, p = 0.007) and characteristic path length (r = -0.686, p = 0.041) at 1 month after stroke.Discussions
Significant increase in the local structural brain connections were observed in brain regions that were largely located in the spared visual and limbic systems at 3 and 6 months after stroke (Figure 1). To perform hand grasping, hemiparetic patients usually relies more on visual feedback to compensate for impaired feed-forward motor control 7. The outgrowth of structural connections from spared network that we observed may thus be a plausible neuroplastic changes 8 to compensate for the impaired motor pathways. Such neuroplasticity has also been previously demonstrated histopathologically 9,10 and from functional brain network 1,11–13.
We also found that the more the global structural brain network is disrupted (i.e. decrease in γ and increase in characteristic path length), the worse is their motor recovery after rehabilitation (Figure 2). In other words, the acute/subacute effect of ischemic subcortical infarct on the global but not local brain network organization has the greatest bearing on motor recovery. Wang et al 1 observed similar association using resting-state fMRI, wherein the γ of the motor executive network was significantly correlated with multiple neurological scales during recovery.
Conclusion
We have successfully demonstrated that reorganization in the structural brain network occurred in the brain regions that were largely located in the spared visual and limbic systems for patients with acute subcortical ischemic infarct in the motor system, likely a compensation mechanism for regaining motor functions. More importantly, our results suggest that structural brain network topology measured acutely/subacutely could potentially be a prognostic indicator of motor recovery for acute stroke patients.Acknowledgements
This work was fully supported by the Research Grants Council of the Hong Kong Special Administrative Region, China, HKU 17108514References
1. Wang L, Yu C, Chen H, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain 2010;133:1224–38.
2. Park C, Chang WH, Ohn SH, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke 2011;42:1357–62.
3. Wang R, Benner T. Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med 2007;15:3720.
4. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–69.
5. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38.
6. Cai J, Ji Q, Xin R, et al. Contralesional Cortical Structural Reorganization Contributes to Motor Recovery after Sub-Cortical Stroke: A Longitudinal Voxel-Based Morphometry Study. Front Hum Neurosci 2016;10:393.
7. Raghavan P, Santello M, Gordon AM, et al. Compensatory Motor Control After Stroke: An Alternative Joint Strategy for Object-Dependent Shaping of Hand Posture. J Neurophysiol 2010;103:3034–43.
8. Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 2009;10:861–72.
9. Zhang ZG, Chopp M. Promoting brain remodeling to aid in stroke recovery. Trends Mol Med 2015;21:543–8.
10. Ueno Y, Chopp M, Zhang L, et al. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke 2012;43:2221–8.
11. Gerloff C, Bushara K, Sailer A, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain 2006;129:791–808.
12. Tombari D, Loubinoux I, Pariente J, et al. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage 2004;23:827–39.
13. Baron J-C, Cohen LG, Cramer SC, et al. Neuroimaging in stroke recovery: a position paper from the First International Workshop on Neuroimaging and Stroke Recovery. Cerebrovasc Dis 2004;18:260–7.
14. Pennisi G, Alagona G, Rapisarda G, et al. Transcranial magnetic stimulation after pure motor stroke. Clin Neurophysiol 2002;113:1536–43.
Figures