4820
Monitoring diabetic stroke response to novel p38 MAPK inhibitor therapy using dynamic contrast enhanced magnetic resonance imaging (DCE-MRI)1Department of Radiology, Zhongda Hospital,Medical School, Southeast University, Nanjing, China
Synopsis
We monitored the increased disruption of blood brain barrier (BBB) by DCE-MRI at acute-stage of ischemic stroke in T2DM mice non-invasively. Furthermore, administration of novel P38 inhibitor is a promising way to promote BBB recovery in diabetic stroke and the therapeutic efficacy can be monitored by DCE-MRI.
In this study, we monitored the increased disruption of blood brain barrier (BBB) by DCE-MRI at acute-stage of ischemic stroke in T2DM mice non-invasively. Furthermore, administration of novel P38 inhibitor is a promising way to promote BBB recovery in diabetic stroke and the therapeutic efficacy can be monitored by DCE-MRI.
Introduction: Type 2 diabetes (T2DM) is an independent risk factors for ischemic stroke and worsen the outcomes. Blood-brain barrier (BBB) damage plays a crucial role in cerebral ischemia and is closely associated functional recovery. Increasing evidence have proven that hyperglycemia in T2DM altered integrity of BBB and in turn promoted BBB disruption in ischemic stroke1. The activation of P38 MAPK in T2DM caused a series of inflammation response which closely associated with the structure of BBB. Measurement and quantitative the increased BBB permeability not only can serve as a diagnostic indicator but also helpful for therapy approach chosen. In this study, we monitored the dynamic change of BBB by DCE-MRI at acute-stage of ischemic stroke in wild-type and T2DM mice non-invasively. Furthermore, T2DM mice were administrated with a novel P38-MAPK inhibitor and examined with DCE-MRI in vivo at following days to detect the therapeutic efficacy.
Methods: 1.Animals: All animal experiments were approved by the Institutional Animal Use Care Committee of Medical School of Southeast University. The C57BL/6J mice were randomly divided into 3 groups: (1) Wild-type mice (2) STZ-induced diabetic mice (3) db/db mice. The middle cerebral artery was occluded by photochemical reaction after photosensitizer injection. 2.MRI: Mice were anesthetized with 1.5%-2% in 20% oxygen and positioned in the 7.0T small animal MR scanner (Bruker PharmaScan, Germany). A 26G catheter was inserted into the tail vein of mice.T1 map on brain was obtained using RARE sequence with TR=3000, 1200, 800,500,400,353ms, flip angles of 15°, 30°, 45°, 60°and 75°map.FOV:2.0×2.0cm, matrix:256 ×256,thickness:1.0mm. The DCE-MRI parameters were TR=100ms, flip angle=308, with other imaging parameters the same as above. Five images were acquired before bolus of 0.1mmol/kg Gd-DTPA was administrated within 30s via the tail vein catheter. Parameters were analyzed in RR model of DCEurLAB software2.
Results:
1. Increased BBB permeability in diabetic mice after ischemic stroke To address the BBB permeability difference between wild-type and diabetic stroke mice, we performed dynamic contrast-enhanced MRI (DCE-MRI) on 24 hours post ischemia. RR models, which can used in pre-clinical settings, provided two independent parameters: blood-to-tissue transfer constant (Ktrans) , extravascular extracellular space faction (Ve). Fig.1A demonstrated the Ktrans map of the ischemic stroke lesion. For diabetic groups, average Ktrans in stroke lesion were higher than wild-type mice. Average Ktrans was determined as 0.089 mL/min/100ml in db/db mice, 0.0395 mL/min/100ml in STZ-induced T2DM mice, and 0.018 mL/min/100ml in wild-type mice (Fig.1B). In addition, Ve fraction presented same trend with Ktrans (Fig.1C).
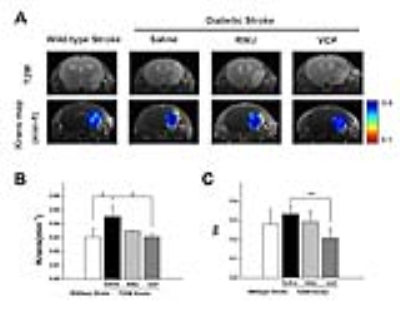
2.Novel P38 MAPK inhibitor decreased BBB permeability in diabetic mice We used a novel P38 MAPK inhibitor-VCP, which has higher affinity to suppress the inflammation response and in turn alleviate the BBB damage. Saline, traditional P38 inhibitor-RWJ and VCP were intragastrically administrated twice a day for consecutive 7 days in STZ-induced diabetic stroke mice. DCE-MRI was performed on day 7 post stroke to evaluate the therapeutic response of P38 inhibition. Figure 2A demonstrated the Ktrans maps, diabetic stroke mice treated with VCP displayed a significantly reduction of Ktrans values compared with saline group(0.030±0.002 vs. 0.0451±0.0078,p=0.012)(Fig.2B). Furthermore, the same trend was observed in ne fraction calculation.
Discussion: T2DM has been proven promoted the BBB injure after stroke. Meanwhile, phosphorylation of p38 MAPK triggers in inflammatory cytokines release in diabetes condition may be a potential reason for BBB damage. Visualizing the dynamic change of BBB in vivo is a promising to predict prognosis and evaluate therapeutic efficacy. Among of the molecular imaging modalities, DCE-MRI has spatial resolution and sensitivity in BBB evaluation. By using DCE-MRI, we found that diabetic mice presented increased permeability of BBB, which indicated worsen brain damage. In addition, DCE-MRI analysis found that Ktrans and ne fraction were both decreased in VCP-treated diabetic stroke mice, suggesting improved BBB recovery.
Conclusion
In conclusion, our finding suggested that the novel p38 inhibitor, VCP promoted BBB recovery in diabetic stroke. Furthermore, DCE-MRI is a promising way to visualize to BBB damage and evaluate the therapeutic efficacy of VCP.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (NSFC, No.81371538, and No.81525014), the Major State Basic Research Development Program of China (973 program, No. 2013CB733802),and the Jiangsu Provincial Special Program of Medical Science(BL2103029).References
1. Poittevin M, Bonnin P, Pimpie C, Rivière L, Sebrié C, Dohan A, et al. Diabetic microangiopathy: Impact of impaired cerebral vasoreactivity and delayed angiogenesis after permanent middle cerebral artery occlusion on stroke damage and cerebral repair in mice. Diabetes. 2015;64:999–1010.
2. Ortuño JE, Ledesma-Carbayo MJ, Simões R V, Candiota AP, Arús C, Santos A. DCE@urLAB: A dynamic contrast-enhanced MRI pharmacokinetic analysis tool for preclinical data. BMC Bioinformatics [Internet]. 2013;14. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84886735382&doi=10.1186%2F1471-2105-14-316&partnerID=40&md5=1d5682f8b47d66b6d77f2a7a71264e16
Figures
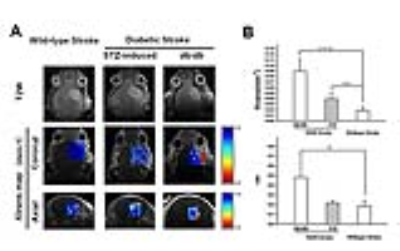
(A)Representative Ktrans maps of ischemic mouse brain. (B)Ktrans values and ve fraction of the infarct lesion.
n=4, Data are mean ± SD. *P < 0.05, **P < 0.01 , ***P < 0.001