4819
Motor recovery after initial severe stroke: confronting kinematics with brain activations1Division of interventional Neuroradiology, I2FH, Gui de Chauliac, Montpellier University Hospital, Montpellier, France, 2Physical Medicine and Rehabilitation, Lapeyronie, Montpellier University Hospital, Montpellier, France, 3EuroMov, University of Montpellier, Montpellier, France, 4Physical Medicine and Rehabilitation, le Grau du Roi, Nimes University Hospital, Nimes, France, 5Division of interventional Neuroradiology, Gui de Chauliac, Montpellier University Hospital, Montpellier, France
Synopsis
To maximize motor recovery of the upper-limb post-stroke, rehabilitation should be adapted to the individual patient. This requires the identification of motor recovery markers in relation to corresponding brain activations. During elbow flexion/extension, kinematic analysis was confronted with corresponding fMRI activations, comparing 21 participants post-stroke with 13 controls. This provided insight into the underlying functioning and organisation of motor control, switching between ‘automatic’ feed-forward and ‘conscious’ feedback control. Post-stroke, the latter strategy was applied with an additional role for visualisation and the contralesional hemisphere, whereby different kinematic profiles were related to different brain activations, opening doors to personalized rehabilitation.
Introduction
Over 50% of people that survive a stroke show important sensorimotor deficits of especially the upper-extremities. Although the paretic upper-limb is most strongly affected, also the ipsilateral upper-limb is marked by altered motor behaviour. Rehabilitative training is essential to guide and favour recovery. Its efficiency depends on underlying neural plasticity processes. To favour plasticity and maximize recovery, rehabilitation should be adapted to the individual patient. Here we use fine-grained kinematics to reveal markers of motor recovery in relation to the corresponding brain activations.Methods
13 healthy controls and 21 participants post-stroke with a first-ever unilateral supra-tectorial ischemic stroke participated in this study. All post-stroke participants had initial severe motor deficits (Fugl-meyer score <30/60). Within 8 weeks post-stroke (V0) and after 6 weeks of rehabilitation (V1) participants performed an elbow flexion/extension task in the fMRI (1.5T) with simultaneous kinematic movement monitoring using the 3D ultrasound motion caption system (Zebris, Medical GmbH). A block-design was used, alternating ten ‘resting’ volumes (both arms stretched alongside the body) with ten ‘active’ (continues flexion/extension of the elbow in the vertical plane). The paradigm was performed separately for each upper-limb, followed by a synchronous bilateral condition. Participants had their eyes closed. Statistic threshold was set at p<0.001, FWE cluster-corrected. Kinematic variable selection was based on previous research demonstrating that movement organization in healthy and post-stroke populations can be captured by the displacement of end-point (amplitude), movement velocity (frequency), smoothness (quantification of submovements) and directness of the trajectory (normalized trajectory length).1 In addition, biological noise makes our movement inherently variable, asking for continuous correction processes. The sample entropy was calculated to gain insight into the structure of this variability, whereby increased entropy is thought to indicate more automatic control processes.2Results/Discussion
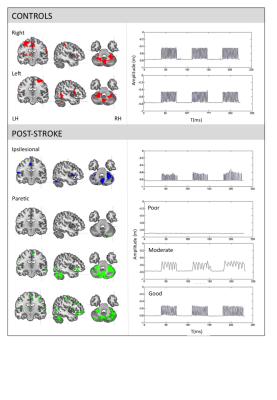
The healthy controls performed a smooth sinusoidal movement with slight variations between cycles. There were no differences in kinematic values between right, left, and bilateral movements. Evidently, differences between conditions were observed post-stroke. However, in general they moved with decreased amplitude, smoothness, directness and entropy. The frequency was lower for the paretic upper-limb and during synchronised bilateral movements, but comparable to controls for the ipsilesional upper-limb (table.1). See fig.1 for task-related activations and movement profiles at V1.
The analysis of the healthy control group suggested that adding kinematic regressors to the design matrix of the fMRI analysis could provide insight into the underlying functioning and organisation of motor control.3 We found the involvement of the posterior cerebellum and middle frontal regions as a function of the entropy of movement variability, and the primary sensoricortex in relation to the trajectory directness. Higher entropy was correlated with a higher frequency, whereas lower directness of the trajectory was related to a lower frequency. It was suggested that different networks were related to two different aspects of rhythmical movement: rhythmicity and error control. The partitioning between more automatic feedforward control involving these cerebellar-frontal circuits, versus less automatic feedback-based control involving the sensory cortex was regarded essential for optimal performance.
Subsequently, focussing on the ipsilesional upper-limb post-stroke, extended activation of the primary sensorimotor cortex (V0) was observed, as well as additional recruitment of the contralesional middle temporal gyrus (V0) and roladic opercularis (V0+V1), and lacking activation of the supramarinal gyrus. Extended activations may indicate motor learning,4 whereas the middle temporal gyrus is related to spatial,5 the rolandic opercularis to movement visualisation,6 and the supramarginal gyrus to priopriocepsis.7 Kinematic movement characteristics were unrelated to clinical scores and lesion characteristics, supporting the idea that they reflect adaptive motor control strategies. The kinematic profile suggested a stronger feedback-based control of the ipsilesional upper-limb. An additional role for visualization during task execution in the absence of reliable proprioception and sight was demonstrated.8
Finally, concerning the paretic upper-limb, three recovery groups were identified: poor, moderate and good recovery. The moderate group showed the strongest variation in brain activity patterns. Nevertheless, again a correlation was identified between the middle frontal regions, the posterior cerebellum and the entropy of movement variability. Also, a correlation between the amplitude of movement and the occipital lobe was observed. Together with activations in the rolandic opercularis, this implies that visualisation of movement was indeed applied as active strategy, especially in the absence of movement.
Conclusion
The co-registration of fine-grained kinematics and fMRI measures, has so far allowed us to demonstrated how different kinematic profiles are related to different motor control organisations. Analysing kinematics in a standardized manner might contribute to the implementation of a personalized rehabilitation strategy, and more targeted brain stimulation protocols to stimulate plasticity and optimize recovery.Acknowledgements
This research was funded by the French 'Ministère de la Santé (MARGAUT: 2010-A00596-33), the Agence nationale de la Recherche (NUMEV: ANR-10-LABX-20) and by the Montpellier University Hospital (AOI-MAPPY: MV/PI/UF9858).References
1 van Dokkum L, Hauret I, Mottet D, et al. The contribution of kinematics in the assessment of upper-limb motor recovery early after stroke. Neurorehabil Neural Repair. 2014;28:4-12.
2 Richman JS, Moorman JR. Physiological time-series using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:2039-2049.
3 van Dokkum LEH, Mottet D, Laffont I, et al. Kinematics in the brain: unmasking motor control strategies? Exp Brain Res. 2017;253:2639-2651.
4 Rao SM, Binder JR, Bandettini PA, et al. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311-2318.
5 Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Science, 1995;270:102-105.
6 Binkofski F, Amunts K, Stephan KM, et al. Broca's Region subserves imagery of motion: a combined cytoarchitectonic and fMRI study. Hum Brain Mapp. 2000;11:273-285.
7 Ben-Shabat E, Matyas TA, Pell GS, et al. The right supramarginal gyrus is important for proprioception in healthy and stroke-affected participants: a functional MRI study. Front Neurol. 2015;6:248.
8 van Dokkum LEH, le Bars E, Mottet D, et al. Modified brain activations of the non-damaged hemisphere during ipsilesional upper-limb movement in persons with initial severe motor deficits post-stroke. Neurorehabil Neural Repair 2017. Epub a-head of print.
Figures
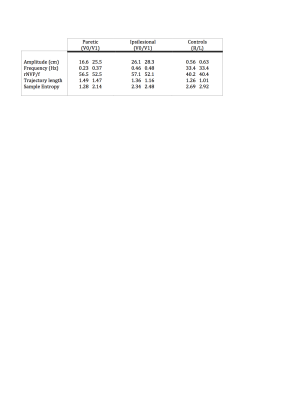
Table.1 Kinematic characteristics of the elbow flexion/extension task.
Overview of mean kinematics for the paretic and ipsilesional upper-limb post-stroke at time-point V0 and V1 and the right (R) and Left (L) upper-limb in controls. rNVP/f= relative number of velocity peaks by the frequency.