4818
A comparison of cerebrovascular reactivity at 1.5 and 3T in cerebral small vessel disease patients1Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom, 2Edinburgh Dementia Research Centre in the UK Dementia Research Institute, Edinburgh, United Kingdom, 3Fondation Leducq Network for the Study of Perivascular Spaces in Small Vessel Disease, University of Edinburgh, Edinburgh, United Kingdom
Synopsis
Cerebrovascular reactivity can be measured using blood oxygen level dependent (BOLD) MRI and is a potential mechanism in cerebral small vessel disease (SVD). Investigations of the effect of field strength on CVR have been limited, particularly in patient groups. In this study CVR measurements within a series of preselected regions in SVD patients were assessed at 1.5 and 3T. Mean CVR was greater at 3T in 12 of the 14 regions, however differences, as assessed with Bland-Altman plots, were within reasonable limits. These results point to the importance of considering other scanner specific factors beyond field strength when measuring CVR.
Introduction
Blood oxygen level dependent (BOLD) sequences have been used as a means of measuring cerebrovascular reactivity (CVR), the change in blood flow in response to a vasoactive stimulus. CVR has been implicated in a number of conditions1,2 and is thought to play a role in small vessel disease (SVD)3,4 where it may relate to impaired vasodilation5. Field strength is known to have an important influence on BOLD contrast, which includes intra- and extravascular components6, with increased field strength being linked to enhanced contrast to noise7,8,9. Comparisons of CVR at 3 and 7T have shown an approximately linear relationship between R2* CVR values and field strength10. However although measurements of CVR have mainly utilised field strengths of 3T or higher, as 1.5T MR scanners remain more common in the clinical setting it is important for comparability to consider the effect of field strength on expected CVR values for patient populations. In this study CVR values are compared between 1.5 and 3T in a group of SVD patients.Methods
Patients with a history of minor ischaemic stroke and radiological evidence of SVD were recruited locally as previously described11,12. For the CVR paradigm 1.5T images were acquired on a GE Signa HDxt MR scanner (General Electric, WI) using a GRE-EPI sequence (TR/TE = 3000/45 ms; 4 mm isotropic voxels). A Siemens Verio MR system (Siemens Healthcare, Germany) was used for acquisition at 3T (TR/TE 3000/30 ms, 3 mm isotropic voxels). A neurovascular structural imaging protocol including T1, T2, FLAIR and GRE scans was also acquired.
The CVR protocol adopted has previously been described13. In brief alternating blocks of medical air (2 minutes) and 6% CO2 (3 minutes) were administered during a 12 minute scan via a unidirectional breathing circuit. A clinician monitored vital signs throughout with end-tidal CO2 being recorded. Fourteen regions of interest (ROIs) affected in SVD were chosen to sample white matter (WM) and subcortical grey matter (GM) before being manually drawn on the 1.5T T1w image. Stroke lesions and areas of blooming around the large veins and venous sinuses were excluded from the masks. ROIs were transformed on to 3T images and manually corrected for errors. CVR analysis was performed using linear regression with a variable delay. Differences in CVR were assessed with Bland-Altman plots and paired t-test used to test for significant differences.
Results
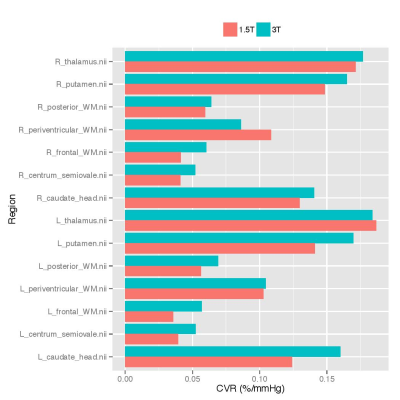
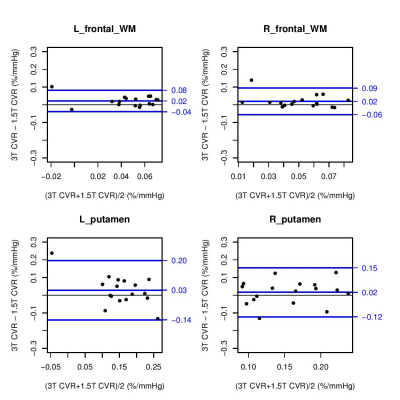
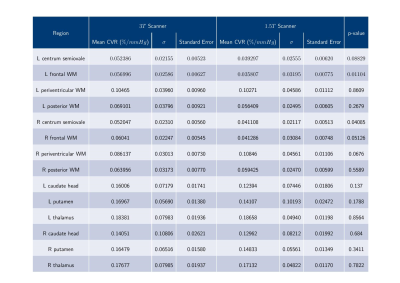
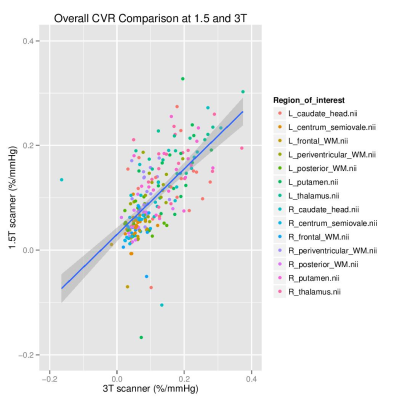
Data was analysed from 17 SVD patients (mean age: 65 years, range: 53-76; 24% female). Mean CVR values were greater at 3 than 1.5T in 12 of the 14 regions considered (Figure 1), though only the left frontal WM (p=0.01104) and right centrum semiovale (p=0.04085) were significantly different (p<0.05). The region-wise Bland-Altman plots for the CVR values (Figure 2) suggest that differences between 1.5 and 3T were within reasonable levels and did not display a systematic bias, though the degree of variability differed across regions. CVR in grey matter exceeded that in white matter at both field strengths (1.5T: 0.124-0.187 vs 0.036-0.108 %/mmHg, 3T: 0.141-0.184 vs 0.052-0.105 %/mmHg, both p<2x10-16) (Figure 4).Discussion
Although there were differences in CVR values measured at 1.5 and 3T these were not as pronounced as those previously reported in comparisons at higher field strength for CVR10 or functional MRI7. This may point to the role of other factors, including scanner specific parameters, or the interval between scans at 1.5 and 3T by which time CVR may have been further impaired. In particular 1.5T scanners can exhibit reduced geometric distortion and signal dropout from susceptibility effects than at higher field strengths14. The manual selection of predetermined regions may also be a factor through limiting the effect of signal contamination associated with tissue boundaries, while venous blooming was less pronounced. The Bland-Altman plots generally showed good agreement between CVR values at 1.5 and 3T. In line with the previous literature13,15 CVR was significantly greater within grey matter than white matter regions.Conclusion
This study highlights the importance of considering field strength amongst others factors, particularly region selection, when measuring CVR. Although CVR values were generally greater at 3T, and significantly so in two regions, differences were small and patterns of regional variation were similar. As such along with field strength other scanner specific considerations, including geometric distortion and signal to noise ratio, should be factored in, particularly for cross-centre studies. Further methodological work is also required to improve comparability, not least through detailed reporting and greater consensus on best practice16. Lastly while this work underscores the viability of measuring CVR in SVD patients at 1.5T, reproducibility should be investigated.Acknowledgements
This work was funded by the
Chief Scientist Office of Scotland (grant ETM/326), the Wellcome Trust-
University of Edinburgh Institutional Strategic Support Fund, NHS Lothian
Research and Development Office (MJT), the Scottish Imaging Network: A Platform
for Scientific Excellence (“SINAPSE”, funded by the Scottish Funding Council
and the Chief Scientist Office of Scotland; GB, radiography staff), and China Scholarship Council/University of Edinburgh (YS), the Alzheimer’s Society (grant ref AS-PG-14-033; GB), the European Union Horizon 2020, ‘‘SVDs@target’’(grant No 666881; MS, GB), Fondation Leducq (grant 16 CVD 05). The Stroke Association Garfield Weston Foundation Senior Lectureship (FD), NHS Research fellowship (FD) and the Medical Research Council (FD). We also thank the radiographers for providing expert research support and the patients for their participation.
References
1. Cantin S, Villien M, Moreaud O, et al. Impaired cerebral vasoreactivity to CO2 in Alzheimer's disease using BOLD fMRI. Neuroimage 2011;58(2):579–587.
2. Dumas A, Dierksen GA, Gurol ME, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol 2012;72(1):76–81.
3. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12(5):483–497.
4. Blair GW, Doubal FN, Thrippleton MJ, et al. Magnetic resonance imaging for assessment of cerebrovascular reactivity in cerebral small vessel disease: a systematic review. J Cereb Blood Flow Metab 2016;36(5):833–841.
5. Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006;37(6):1391–1398.
6. Silvennoinen M J, Clingman C S, Golay X, et al. Comparison of the Dependence of Blood R2 and R* 2 on Oxygen Saturation at 1.5 and 4.7 Tesla. Magn Reson Med. 2003;49(1):47-60.
7. Gati J S, Ravi R S, Ugurbil K et al. Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn Reson Med. 1997;38(2):296-302
8. Kruger G, Kastrup A, and Glover G H. Neuroimaging at 1.5 T and 3.0 T: Comparison of Oxygenation-Sensitive Magnetic Resonance Imaging. Magn Reson Med. 2001:45(4):595–604
9. Triantafyllou C, Wald L L, Hoge R D. Echo-Time and Field Strength Dependence of BOLD Reactivity in Veins and Parenchyma Using Flow-Normalized Hypercapnic Manipulation. PLoS ONE 2011;6(9):e24519.
10. Driver I, Blockley N, Fisher J et al. The change in cerebrovascular reactivity between 3 T and 7 T measured using graded hypercapnia. Neuroimage 2010;51(1):274-279
11. Heye AK, Thrippleton MJ, Armitage PA, et al. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. Neuroimage 2016;125:446–455.
12. Wardlaw JM, Doubal F, Armitage P, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol 2009;65(2):194–202.
13. Thrippleton M J, Shi Y, Blair G et al. Cerebrovascular reactivity measurement in cerebral small vessel disease: Rationale and reproducibility of a protocol for MRI acquisition and image processing. Int J Stroke. 2017 Jan 1:1747493017730740.
14. Dietrich O, Reiser MF, Schoenberg SO. Artifacts in 3-T MRI: Physical background and reduction strategies. Eur J Radiol 2008;65(1):29-35
15. Thomas BP, Liu P, Park DC et al. Cerebrovascular reactivity in the brain white matter: magnitude, temporal characteristics, and age effects. J Cereb Blood Flow Metab 2014;34(2):242-247.
16. Bright M, Mazerolle EL, Sobczyk O et al. Clinical mapping of cerebrovascular reactivity using MRI: a framework for reaching consensus. Proc ISMRM 2017;1666.
Figures