4815
Increased intracellular volume fraction, orientation dispersion and diffusion kurtosis in the brain are associated with poor functional outcome in comatose cardiac arrest patients1Department of Radiology, MGH Athinoula A Martinos Center, Charlestown, MA, United States, 2Department of Neurology, Massachusetts General Hospital, Boston, MA, United States, 3Department of Anesthesiology, Massachusetts General Hospital, Boston, MA, United States, 4Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 5Department of Rehabilitation Neuropsychology, Spaulding Rehabilitation Hospital, Charlestown, MA, United States, 6Department of Neurology, Boston Medical Center, Boston, MA, United States
Synopsis
Cardiac arrest patients who are comatose after restoration of spontaneous circulation were prospectively studied to determine whether changes to intracellular volume fraction (ICVF), orientation dispersion and diffusion kurtosis imaging (DKI) can be used to discriminate patients likely to recover consciousness. Subjects who failed to wake up had greater median ICVF, and DKI compared to subjects who woke up. Increases in ICVF, and DK are associated with more severe acute ischemic brain injury. Multi-shell diffusion imaging may help identify patients that may recover consciousness.
Introduction
For cardiac arrest (CA) survivors initially comatose after restoration of spontaneous circulation (ROSC), the extent of brain injury and expected neurologic outcome are crucial for patient management decisions.1 Critical knowledge gaps persist in neuroprognostication of comatose post-CA survivors.2 Early prognostication is difficult except in the extremes of cases: patients rapidly awakening do well, and those with minimal brain function do poorly. Most patients fall between these extremes. Several studies have also shown that large reductions in the apparent diffusion coefficient were predictive of poor outcome.3, 4 We sought to determine whether multi-shell diffusion imaging techniques such as neurite orientation dispersion and density imaging (NODDI)5 and diffusion kurtosis imaging (DKI)6 can provide additional insight into tissue integrity and potential for recovery of consciousness complementary to standard diffusion tensor imaging (DTI).Methods
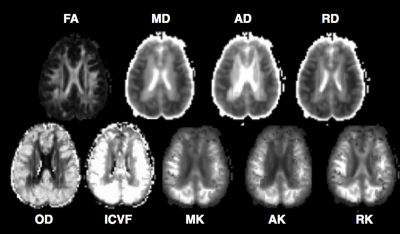
CA patients who were comatose after restoration of spontaneous circulation were prospectively enrolled. Coma was defined as Glasgow Coma Scale (GCS) <=8. All subjects underwent 3T MRI. High-spatial resolution 3D T1-weighted anatomical images were acquired for registration purposes with FOV=256x256 mm2, acquisition matrix=256x256, 176 sagittal slices (thickness 1 mm). Multiple shell diffusion imaging was acquired using 30 directions with b-value=1000 s/mm2, and 2000 s/mm2 (3x3x3 mm3), and 10 b-value=0 s/mm2 images acquired using blipped simultaneous multi-slice7 echo planar imaging (EPI). Neurite orientation dispersion (OD),8 intracellular volume fraction (ICVF),8 mean kurtosis (MK),9 axial kurtosis (AK),9 radial kurtosis (RK),9 mean diffusivity (MD),9 axial diffusivity (AD),9 radial diffusivity (RD)9 and fractional anisotropy (FA)9 were calculated. All images were coregistered to the ICBM MNI 152 1 mm atlas. Using the ICBM probabilistic atlases,10 probability masks for the following regions were generated: frontal, insula, occipital, parietal and temporal lobes; caudate, putamen, and thalamus using a threshold of 50%. Median values in these regions and the whole brain from subjects with poor outcomes (no arousal recovery [AR] by discharge) were compared with those with AR. Univariate logistic regression were used for statistical analysis.Results
Eighteen subjects (mean ±SD 48±23 y, 39% men) were prospectively enrolled. Median (range) post-ROSC GCS was 3 (3-5). 10 patients exhibited AR, 8 did not. 4 subjects had good 6 month outcome. Median [IQR] time-to-MRI was 5 [4-8] days. Neither age, sex, nor GCS were predictive of AR. On univariate analysis, whole brain decreased RD (p=0.024), increased MK (p=0.025), increased RK (p=0.046), and increased ICVF (p=0.022) were predictive of AR. Regional analysis showed that reduced MD in the occipital (p=0.015), and parietal (p=0.028) lobes; reduced AD in occipital (p=0.015), and parietal (p=0.028) lobes; reduced RD in occipital (p=0.015), and parietal (p=0.027) lobes; increased MK in frontal (p=0.042), occipital (p=0.019), parietal (p=0.024), and temporal (p=0.043) lobes; increased AK in frontal (p=0.040), occipital (p=.017), parietal (p=0.023), and temporal (p=0.040) lobes; increased RK in frontal (p=0.042), occipital (p=0.021), parietal (p=0.025), and temporal (p=0.049) lobes; increased OD in occipital (p=.017), parietal (p=0.028), and temporal (p=0.040) lobes; and increased ICVF in frontal (p=0.021), insula (p=0.029), occipital (p=0.013), parietal (p=0.016), and temporal (p=0.022) lobes, putamen (p=0.038), and white matter (p=0.032) were associated with failed arousal recovery.Discussion
Patients who failed to exhibit arousal recovery had greater whole brain mean kurtosis and radial kurtosis and ICVF values, but lower radial diffusivity compared to patients who woke up. Regions of the brain that were particularly affected were occipital and parietal lobes. Increased ICVF is suggestive of more extensive cytotoxic edema whereas increased OD might reflect greater loss of structural integrity in patients who do not wake up. Differences in timing of MRI acquisition, potential bias from self-fulfilling prophecy and small sample sizes are limitations of our findings. Although multi-shell diffusion findings were not made available to the clinical team, the clinical MRI data that were shared may have influenced treatment decisions.Conclusions
In addition to reductions in diffusivity, alterations in ICVF, OD and kurtosis measures may provide insight in understanding the pathophysiological mechanisms underlying disorders of consciousness and play an important role in guiding patient management decisions in comatose cardiac arrest patients.Acknowledgements
We thank Drs. Himanshu Bhat, Dylan Tisdall, Andre van der Kouwe, Kawin Setsompop and Steven Cauley for providing pulse sequences that were used in this study.References
1. Dragancea I, Rundgren M, Englund E, Friberg H, Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation. 2013;84:337-342
2. Neumar RW, Shuster M, Callaway CW, Gent LM, Atkins DL, Bhanji F, Brooks SC, de Caen AR, Donnino MW, Ferrer JM, Kleinman ME, Kronick SL, Lavonas EJ, Link MS, Mancini ME, Morrison LJ, O'Connor RE, Samson RA, Schexnayder SM, Singletary EM, Sinz EH, Travers AH, Wyckoff MH, Hazinski MF. Part 1: Executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S315-367
3. Wu O, Sorensen AG, Benner T, Singhal AB, Furie KL, Greer DM. Comatose patients with cardiac arrest: Predicting clinical outcome with diffusion-weighted MR imaging. Radiology. 2009;252:173-181 4. Wijman CA, Mlynash M, Caulfield AF, Hsia AW, Eyngorn I, Bammer R, Fischbein N, Albers GW, Moseley M. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann Neurol. 2009;65:394-402
5. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. Noddi: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000-1016
6. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432-1440
7. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magnetic resonance in medicine. 2012;67:1210-1224
8. Daducci A, Canales-Rodriguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran JP. Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. Neuroimage. 2015;105:32-44
9. Tabesh A, Jensen JH, Ardekani BA, Helpern JA. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med. 2011;65:823-836
10. Laboratory of Neuro Imaging UCLA. ICBM 452 T1 atlas. 2008;2007