4814
New vision of tuberous sclerosis complex on 7-Tesla MRI1State Key Laboratory of Brain and Cognitive Science, Beijing MRI Center for Brain Research, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, 2University of Chinese Academy of Sciences, Beijing, China, 3Chinese PLA general hospital, Beijing, China, 4Beijing Tian Tan Hospital, Capital Medical University, Beijing, China
Synopsis
Tuberous sclerosis complex is a multisystem genetic disorder characterized by the growth of numerous tuberous lesions in brain. However, few in vivo studies on TSC have focused on venous structure changes, their association with TSC lesions, and iron accumulation in basal ganglia. 7T susceptibility weighted imaging was performed on eleven TSC patients in comparison with fifteen age- and sex-matched healthy controls. The tubers might develop along penetrating veins. There might be coexistence of iron deposition and calcification in basal ganglia. These in vivo 7T MRI findings provided new perspectives for better understanding the brain pathology in patients with TSC.
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder resulted from mutations of TSC1 or TSC2 genes1. In brain, TSC are implicated in cell body size, dendritic arborization, axonal outgrowth and targeting, neuronal migration, cortical lamination, spine formation and vascular abnormalities. However, few in vivo studies on TSC have focused on venous structure changes, their association with TSC lesions, and iron accumulation in basal ganglia. This may hinder the study on the lesion development and pathogenesis of TSC. In this study, in vivo 7T SWI was utilized to explore these fields.Methods
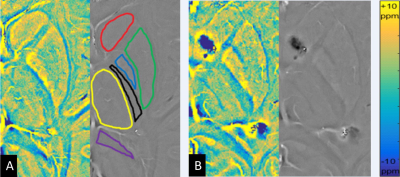
Eleven TSC patients (8-36 years, 5 males) were scanned at a 7T MRI system (Siemens, Erlangen, Germany) with a volume transmit/32 channel receiver head coil (Nova Medical, USA). Beyond T1-, T2-weighted and FLAIR images, other high-resolution images were also acquired. 3D white matter suppressed (WMS) MPRAGE was included to improve the TSC lesion detection with the following parameters: field of view=230*230mm2, spatial resolution=0.7*0.7*0.7mm3, echo/repetition/inversion time=3.38/3000/580ms, scan time=5.8minutes; 3D SWI: field of view=221*200mm2, spatial resolution=0.3*0.3*2 or 2.5mm3, echo/repetition time=15/23ms, scan time=6minutes. Age- and sex-matched healthy controls were also scanned with the SWI protocol. WMS MPRAGE subdued the signal of white matter to make the tubers stand out. SWI was utilized to analyze vascular abnormalities, their relationship with TSC lesions and iron accumulation in basal ganglia. The study was approved by the Institutional Review Board of the Beijing MRI Center for Brain Research. In order to quantify iron accumulation in the basal ganglia, high-resolution phase images of SWI were converted to LFS maps2. Regions of interest (ROIs) were semi-automatically drawn in basal ganglia, including caudate, putamen, thalamus and globus pallidus (Fig. 1). Regions of the splenium of the corpus callosum and the posterior internal capsule were also extracted as references. Phase images (measured in radians) were firstly quantified and then transformed to LFS maps (measured in parts per billion, ppb) by subtracting the mean phase of the posterior internal capsule and dividing by (2π*TE*γ*B0). The final LFS value for brain structure in each subject was calculated by averaging the pixel values of the ROI. The correlation between LFS and TSC disease duration (age of TSC patient) was analyzed. The mean LFS in TSC patients were compared with healthy controls using multiple linear regression analysis.Results
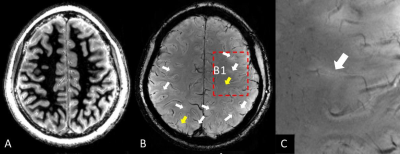
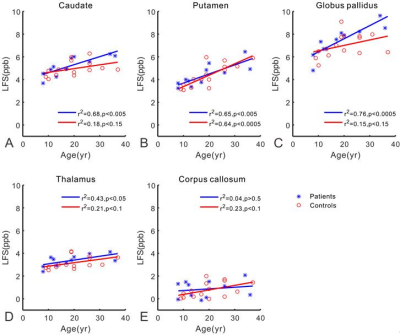
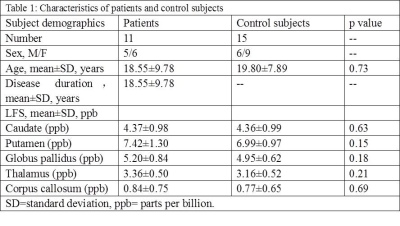
Clinical data of eleven TSC patients and fifteen healthy control subjects were summarized in Table 1. WMS protocol at 7T subdued the signal of white matter selectively and revealed tubers in the TSC patients. The tubers demonstrated abnormal grayscale values. Cortical tubers showed a strict perivascular distribution at 7T SWI (Fig. 2). The elongated ones followed the form and orientation of the vessels. It was speculated that tubers developed along vessels. In five of the eleven patients, a total of 123 tubers were identified and 114 (92.7%) of them existed with the company of vessels. The remaining six patients were excluded from this statistics, because some tubers were interlaced with each other and hindered the analysis of one-to-one relationship of tubers and vessels. As indicated in Fig. 3, LFS in basal ganglia was significantly correlated with TSC disease duration (e.g. r2=0.76, p<0.0005 for globus pallidus). There was no correlation between LFS and disease duration in splenium of the corpus callosum. LFS in the basal ganglia of healthy controls have the tendency of increasing with age (P<0.15). Compared with healthy controls, the mean LFS values in basal ganglia of TSC patients were larger, although the differences were not significant (Table 1). The positive and negative LFS values of TSC patients were irregularly distributed in basal ganglia, especially at putamen (Fig. 1). This was possibly due to the coexistence of iron deposition in basal ganglia with calcification, which was diamagnetic and confounded the LFS value.Discussions and Conclusions
7T WMS MPRAGE with new tissue contrast provided a precise delineation of morphological details of tubers. With the increase of field strength, the in vivo 7T SWI had submillimeter spatial resolution and showed high magnetic susceptibility effects, which enabled detecting very small veins and their association with TSC lesions. Phase information was transformed to LFS maps and made it possible to quantify the iron accumulation in the deep basal ganglia. In summary, 7T MRI provided a new vision of TSC by delineating the association of tubers with veins and iron accumulation in basal ganglia. This might have great significance for assessing TSC disease severity, studying pathological process in vivo and helping the development of new therapeutic strategies.Acknowledgements
We thank Ms. Jing An (Siemens Shenzhen MR Ltd. China) for her technical assistance. This work was supported in part by the Ministry of Science and Technology of China (MOST) grants (2015CB351701, 2012CB825500), National Nature Science Foundation of China grant (91132302, 81771388), and Chinese Academy of Sciences Strategic Priority Research Program B grants (XDB02010001, XDB02050001).References
1. Crino PB, Nathanson KL, Henske EP (2006) The tuberous sclerosis complex. N Engl J Med 355:1345–56.
2. Hammond KE, Metcalf M, Carvajal L, et al (2008) Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol 64:707–713.
Figures