4813
Effects of physical exercise on hippocampal volume and vasculature in young adults1Department of Radiology and Nuclear Medicine, Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands, 2Amsterdam Brain and Cognition, University of Amsterdam, Amsterdam, Netherlands, 3Swammerdam Institute for Life Sciences, Center for Neurosciences, University of Amsterdam, Amsterdam, Netherlands, 4Department of Medical Radiation Physics, Lund University, Lund, Sweden, 5C.J. Gorter Center for High Field MRI, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 6Spinoza Centre for Neuroimaging, Royal Netherlands Academy of Arts and Sciences (KNAW), Amsterdam, Netherlands
Synopsis
The underlying neurobiological changes of exercise-induced hippocampal volume increases are poorly understood, but a substantial role for vascular
Introduction
Physical exercise is a promising lifestyle intervention that can improve cognitive functions and has been associated with increases in hippocampal volume1. However, the neurobiological changes underlying these changes are poorly understood. In addition to potential increases in adult neurogenesis, it has been suggested that the hippocampus also exhibits plasticity at the vascular level, including changes in perfusion and angiogenesis2,3. A study in older adults demonstrated that vascular plasticity is provoked by aerobic, but not toning exercise3. It is still unclear whether these effects also occur in young adults, or specifically in older adults. Therefore, our aim was to assess the influence of high (HI) versus low intensity (LI) physical exercise on the hippocampus in young healthy volunteers. Based on previous research2,3, we hypothesized that relative to LI, HI exercise would increase hippocampal volume, as a result of increased vascular plasticity, measured with cerebral blood volume (CBV) and cerebral blood flow (CBF).
Methods
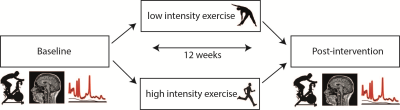
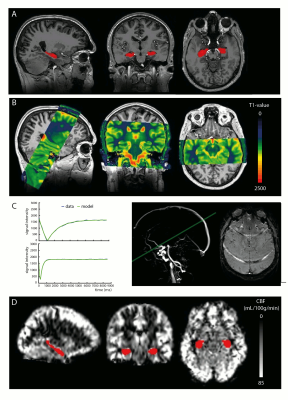
In a double-blind controlled trial (NEUROSHAPE), 52 young healthy controls (mean age=23.5 years) were randomized to either 12 weeks of HI (aerobic) or LI exercise training (stretching and toning). Subjects underwent structural and perfusion MRI assessments and performed a VO2max test, both at pre and post intervention (Figure 1). High-resolution T1-weighted (T1w) scans were obtained on a 7T Philips MR-scanner using a 3D-MPRAGE sequence with the following parameters: TR/TE=4.1/1.8ms, TI=1300ms; voxel size=0.9mm3, FOV=219x240x180mm, flip-angle=7°. Hippocampal volume was calculated from segmentations obtained using the hippocampal longitudinal pipeline implemented in Freesurfer4 (Figure 2A). CBF and CBV measurements were acquired on a 3T Philips MR-scanner. CBV was calculated from the T1 values of tissue and blood before and after contrast administration (0.12mL/kg, 5mL/s, Gadovist) according to the Bookend method5. To obtain hippocampal T1 values (Figure 2B), T1 mapping was performed using a coronal 3D Look-Locker sequence6 covering the length of the hippocampus: TR/TE=10s/4ms, ΔTI=200ms, flip-angle=5°, voxel size=1.1x1.1x2.5mm, FOV=220x220x52mm. T1 blood was measured in a 2mm slice perpendicular to the posterior sagittal sinus (Figure 2C) using a Look-Locker readout6: TR/TE=110/16ms, TI1=25ms, ΔTI=110ms, flip-angle=95°, in-plane resolution=1.5x1.5mm, FOV=230x230mm. CBF was measured using pCASL7: TR/TE=4100/16ms, post-label delay=1525ms, label duration=1650ms, voxel size=3x3x5mm, FOV=240x240x110mm, 50 control-label pairs. Additionally, an M0 scan was obtained. CBF was calculated with a single compartment model8 using the ExploreASL toolbox9 (Figure 2D). In short, T1w images were segmented into gray matter (GM) and white matter (WM) probability maps. Motion was estimated and motion spikes were excluded. Perfusion-weighted images were rigid-body registered to the GM images. The GM and WM maps were spatially normalized using DARTEL to transform the CBF maps to MNI space. The hippocampal mask was extracted from the Harvard-Oxford Atlas and eroded (threshold=10). Repeated measures ANOVA with exercise group (LI/HI) or VO2max change (increase (IVO2) or decrease (DVO2)) as between-subject factor and time (pre and post exercise) as within-subject factor, were used to test the change over time. Correlation analyses were conducted using Pearson’s correlation coefficient.
Results
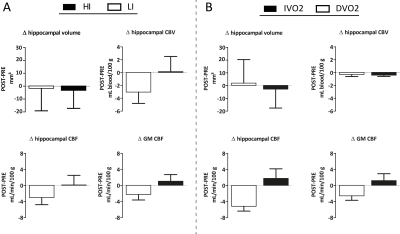
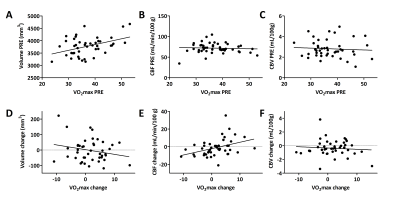
As expected, the HI group improved significantly in VO2max (mean change: 2.28mL/kg/min, p=0.047), while the LI group did not change (mean change: 0.94mL/kg/min, p=0.42). However, the interaction between time and group was not significant (F(43)=0.72, p=0.4). In contrast to our hypothesis, we did not find differences between exercise groups in hippocampal volume and vascular plasticity: no time*exercise group effect was present on volume, CBF or CBV (Figure 3A). However, when splitting the group into those that improved in fitness (IVO2) and those that did not (DVO2), a significant time*group interaction effect was found for hippocampal CBF (F(42)=6.58, p=0.01) and a trend for total GM CBF (F(42)=3.8, p=0.06)(Figure 3B), but not for hippocampal volume and CBV. This was confirmed by a significant association between the change in VO2max and the change in hippocampal CBF (r=0.38, p=0.01)(Figure 4D-E). No association between change in VO2max and change in hippocampal volume or CBV was found. Interestingly, baseline VO2max correlated significantly with baseline hippocampal volume (r=0.42, p<0.01), but not with CBV or CBF (Figure 4A-C).Discussion and Conclusion
Contrary to prior research in older adults, we did not find that exercise increases hippocampal volume in young adults, despite a significant correlation between VO2max and volume at baseline. This could be due to a ceiling effect of neurogenesis and angiogenesis in the healthy young hippocampus. In line with previous studies, we found a significant correlation between exercise-induced changes in cardiovascular fitness and CBF, however, there was also a trend found in total GM CBF. This may suggest that perfusion effects are not limited to the hippocampus, but affect also other brain areas, or that they are the result of systemic cardiovascular effects of exercise.
Acknowledgements
We would like to thank Henk-Jan Mutsaerts for the help with the pCASL processing pipeline.References
- Erickson, K I, Voss, M W, Prakash, R S, et al. Exercise training increases size of hippocampus and improves memory. PNAS, 2010;108(7):3017–3022.
- Pereira, A C, Huddleston, D E, Brickman, A M, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. PNAS. 2006;104(13):5638–5643.
- Maass, A, Düzel, S, Goerke, M, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular Psychiatry. 2015;20(5):585-93.
- Iglesias, J E, Van Leemput, K, Augustinack, J et al. Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject-specific atlases. NeuroImage. In press. 2017
- Sakaie K E, Shin W, Curtin K R, et al. Method for improving the accuracy of quantitative cerebral perfusion imaging. J Magn Reson Imaging. 2005;21(5):512–519.
- Lindgren, E, Wirestam, R, Markenroth, B, et al. Absolute quantification of perfusion by dynamic susceptibility contrast MRI using Bookend and VASO steady-state CBV calibration: a comparison with pseudo-continuous ASL. Magma. 2014;27:487–499.
- Alsop, D C, Detre, J A, Golay, X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine.2015;73(1):102–116.
- Varela, M, Hajnal, J V, Petersen, E T, et al. A method for rapid in vivo measurement of blood T1. NMR in Biomedicine. 2011;24(1):80–8.
- Mutsaerts, H, Thomas, D, de Vita, E, et al. Addressing multi-centre image registration of 3T arterial spin labeling images from the GENetic Frontotemporal dementia Initiative (GENFI). Singapore: ISMRM 2016.
Figures