4810
Surveillance of unruptured intracranial saccular aneurysms using non-contrast 3D black blood MRI: comparison of 3D TOF and CE-MRA with DSA1Radiology, University of California, San Francisco, San Francisco, CA, United States, 2Radiology, Changhai Hospital, Shanghai, China
Synopsis
Patients with unruptured intracranial aneurysms (UIAs) routinely undergo surveillance imaging to monitor the growth. CTA and CE-MRA provide good accuracy in measuring size relative to gold standard 3D DSA, but require contrast agent and/or have radiation, which is undesirable for repeated imaging. We compared three MRI techniques on 58 aneurysms: 1) 3D non-contrast black blood MRI (SPACE), 2) 3D TOF 3) CE-MRA, against gold standard 3D DSA. SPACE was in excellent agreement with DSA, better than CE-MRA and TOF. Our results support the use of non-contrast SPACE for surveillance of UIA in the clinical setting.
Introduction
Intracranial aneurysms (IA) is fairly common with approximately 3% of adults having an unruptured IA (UIA). They have an associated morbidity and mortality due to risk of rupture and the resultant subarachnoid hemorrhage. Clinical management of UIA is based on aneurysm size and location. Because intervention-associated risks may exceed the low rupture rates for smaller UIAs 1, the majority of those patients are followed with surveillance imaging. 3D rotational DSA is the gold standard for IA morphology measurements, but is invasive. CTA and CE-MRA are alternative modalities that show good accuracy compared with DSA. However, they are undesirable for repeated imaging because of radiation and/or the use of contrast agent. 3D non-contrast TOF is another option, however it is subject to flow related artifacts. 3D non-contrast black blood MRI (SPACE) has high isotropic resolution (up to 0.5mm isotropic) with good visualization of the aneurysm geometry and also provides evaluation of the aneurysm wall2. However, it has not been validated against DSA for aneurysm size measurements or compared with clinical MRA techniques. This study aims to compare black blood MRI with 3D TOF/CE-MRA for aneurysm size measurements, using 3D DSA as a reference standard.Methods
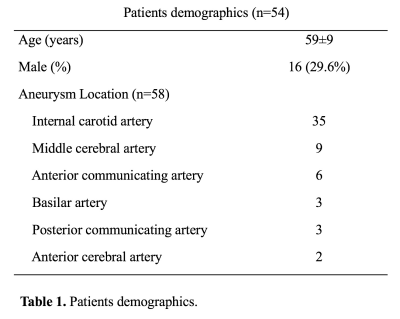
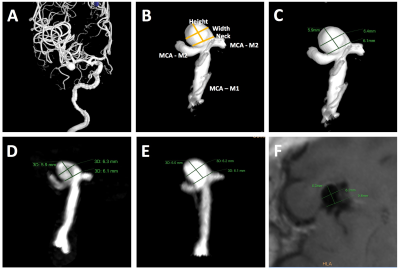
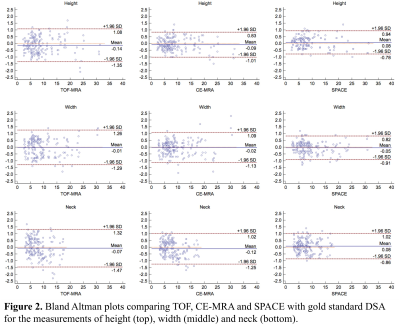
Study population: 54 patients with 58 unruptured intracranial saccular aneurysms were included in the analysis. Patients demographic data and aneurysm locations are shown in Table 1. All patients were scanned in a Siemens 3T scanner (Skyra) using the standard head coil. Sequences: 1) Clinical 3D TOF acquired in axial slab (FLASH), with slice thickness 0.7mm, in plane resolution of 0.5mm, scan time 4’56”. 2) Clinical CE-MRA (FLASH) acquired in coronal plane using first pass with Gd-DTPA, isotropic resolution of 0.7mm, scan time 30 seconds. 3) 3D black blood SPACE (variable flip angle fast-spin-echo 2) acquired in sagittal plane, 0.5mm isotropic resolution, echo train length 60, TR/TE = 900/5.6ms, scan time 8’16”. 4) 3D rotational DSA was acquired following clinical protocol with a 5-second rotation of 200° (144 frames), FOV 32cm and matrix 1024, resulting in 0.3mm in-plane resolution. Image analysis: Two radiologists independently measured the height, width and neck of the aneurysm on the three MRI sequences (MIP was used for TOF and CE-MRA, MPR and MinIP were used for SPACE) and 3D DSA (using volume rendering) (Figure 1). Bland Altman plots were used to compare MRI sequences with DSA. Measurement error was quantified using the coefficient of variance (CV, between measurements SD/mean).Results
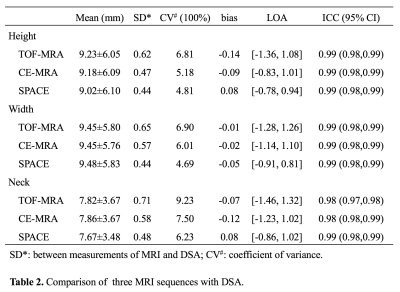
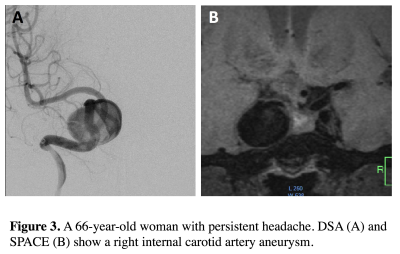
The comparison of three MRI sequences with DSA for aneurysm size measurements is shown in Table 2. Bland Altman plots are shown in Figure 2. 3D SPACE is in best agreement with DSA, with the smallest limit of agreement (LOA) and measurement error (CVs range 4.69% to 6.23%). 3D TOF had the largest LOA and measurement error (CVs range 6.81% to 9.23%). The average CV was 5.24% for SPACE, 6.23% for CE-MRA and 7.65% for TOF. No significant bias was found between the three MRI sequences and DSA. As shown in the BA plots, measurement error didn’t correlate with aneurysm size. Sample images of a patient with a large ICA aneurysm are shown in Figure 3. All three MRI sequences had excellent inter-observer agreement (ICCs>0.95).Discussion
To our knowledge, this is the first study validating 3D black blood MRI against the gold standard DSA for aneurysm morphology measurements. We found 3D SPACE had better accuracy than CE-MRA or 3D TOF. 3D TOF had the poorest agreement with DSA, in agreement with previous studies 3. The accuracy of 3D TOF decreases with increasing aneurysm size, when recirculating and slow flow are commonly present. Good accuracy of CE-MRA has been previously reported compared to DSA 3. In our study, we found SPACE was even slightly better than CE-MRA, possibly due to a higher resolution of SPACE (0.5mm isotropic vs. 0.7mm isotropic of CE-MRA). Our results support the use of non-contrast black blood MRI to replace CE-MRA for the monitoring of intracranial aneurysms. Considering the recent concerns of Gadolinium brain and bone deposition, non-contrast techniques have high potential as a tool for aneurysm surveillance. SPACE also provides the aneurysm wall evaluation, which is a unique advantage. Vessel wall features have been studied as potential markers aneurysm rupture4.Conclusion
3D black blood MRI achieves better accuracy for intracranial aneurysm size measurements than 3D TOF and CE-MRA, using 3D DSA as a gold standard. This non-contrast technique is promising for clinical surveillance of patients with unruptured intracranial aneurysms.Acknowledgements
No acknowledgement found.References
1. Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr., Piepgras DG, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110
2. Zhu C, Haraldsson H, Tian B, Meisel K, Ko N, Lawton M, et al. High resolution imaging of the intracranial vessel wall at 3 and 7 t using 3d fast spin echo mri. Magma. 2016;29:559-570
3. Cirillo M, Scomazzoni F, Cirillo L, Cadioli M, Simionato F, Iadanza A, et al. Comparison of 3d tof-mra and 3d ce-mra at 3t for imaging of intracranial aneurysms. European journal of radiology. 2013;82:e853-859
4. Edjlali M, Gentric JC, Regent-Rodriguez C, Trystram D, Hassen WB, Lion S, et al. Does aneurysmal wall enhancement on vessel wall mri help to distinguish stable from unstable intracranial aneurysms? Stroke. 2014;45:3704-3706
Figures